Stemfactor™ BMP-4, Human Recombinant
03-0007
Brand: Stemfactor
BMP-4 is involved in tooth and limb development and fracture repair, and is a critical signaling molecule required for the early differentiation of the embryo and establishment of a dorsal-ventral axis.
Currency:
| Product name | Product code | Pack size | Price | Price (USD) | Price (GBP) | Price (EUR) |
|---|---|---|---|---|---|---|
| Stemfactor™ BMP-4, Human Recombinant | 03-0007 | 10 μg | (select above) | $ 266.00 | £ 218.00 | € 255.00 |
Note: prices shown do not include shipping and handling charges.
Product Information
Bone Morphogenetic Protein 4 (BMP-4) is a polypeptide belonging to the TGFβ protein super-family. BMP-4 is involved in bone and cartilage development; more specifically, in tooth and limb development fracture repair1. In human embryonic development, BMP-4 is a critical signaling molecule required for the early differentiation of the embryo and establishment of a dorsal-ventral axis2,3. BMP-4 plays an important role in the differentiation of overlying ectodermal tissue. Inhibition of the BMP-4 signal causes the ectoderm to differentiate into the neural plate. In cultured stem cells, BMP-4 plays a distinct role in mouse and human embryonic stem (ES) cells. BMP-4 supports LIF as a positive factor for mouse ES cell self-renewal4. In contrast, BMP-4 induces extra-embryonic trophoblast differentiation in human pluripotent stem cells5. Stemfactor BMP-4 is a recombinant protein expressed and purified from human 293 cells as a glycosylated homodimer with a molecular mass of 34 kDa.

Stemgent and the Stemfactor brand name are trademarks of REPROCELL Inc., Japan.
Product Name: Stemfactor BMP-4, Human Recombinant
Catalog Number: 03-0007
Size: 10 µg
Purity: Greater than 95% by SDS-PAGE analysis.
Formulation: Lyophilized from sterile-filtered 1 M NaCl, 50 mM NaOAc, pH 4.5.
Reconstitution:
- 2019 (Lot J1809-04) or earlier: Centrifuge briefly and then reconstitute BMP-4 in 4 mM HCl to yield a stock solution of no less than 0.1 mg/mL. Avoid freeze-thaw cycles as they can result in loss of activity.
- 2020 (Lot J1912-05) or later: Centrifuge vial briefly before opening. Suspend BMP-4 in sterile water by gently pipetting water down the sides of the vial. DO NOT VORTEX. Allow several minutes for complete suspension. For prolonged storage, dilute to a working dilution in 0.1% BSA in water, divide into single-use aliquots, and store at -80 °C. Avoid repeated freeze-thaws.
Storage and Stability: Stemfactor BMP-4 is shipped at room temperature. Lyophilized BMP-4 is stable for up to 6 months from date of receipt when stored at −20 °C to −80 °C.
- 2019 (Lot J1809-04) or earlier: Reconstituted BMP-4, at concentrations greater than or equal to 0.1 mg/mL, is stable for up to 3 months when stored at −20 °C and up to 6 months when stored at −80 °C.
- 2020 (Lot J1912-05) or later: Reconstituted solutions in water are stable for 1 month at 4 °C. Working dilutions in BSA solution are stable for 3 months at -20 °C to -80 °C.
Sterility: Tested to be negative for Mycoplasma sp. by PCR and microbial contamination by a sterility test.
Source: Stemfactor BMP-4 was expressed in and purified from human 293 cells.
Amino Acid Sequence: SPKHHSQRAR KKNKNCRRHS LYVDFSDVGW NDWIVAPPGY QAFYCHGDCP FPLADHLNST NHAIVQTLVN SVNSSIPKAC CVPTELSAIS MLYLDEYDKV VLKNYQEMVV EGCGCR
Uniprot Accession Number: P12644, residues 293-408
Endotoxin Level: Less than 1.0 EU/µg of BMP-4 as determined by the LAL method.
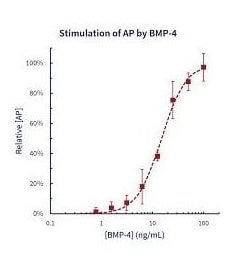
Biologic Activity: The ED50 is less than 30 ng/mL as determined by its ability to induce alkaline phosphatase production by mouse chondrogenic ATDC-5 cells.
Intended use: This product is intended for research use only, and is not for therapeutic or diagnostic use in humans.
- Miljkovic, N.D., Cooper, G.M., and Marra, K.G. Chondrogenesis, bone morphogenetic protein-4 and mesenchymal stem cells. Osteoarthritis Cartilage 16: 1121-1130 (2008).
- Chen, D., Zhao, M., and Mundy, G.R. Bone morphogenetic proteins. Growth Factors 22: 233-241 (2004).
- Sadlon, T.J., Lewis, I.D., and D'Andrea, R.J. BMP4: its role in development of the hematopoietic system and potential as a hematopoietic growth factor. Stem Cells 22: 457-474 (2004).
- Ying, Q.L., Nichols, J., Chambers, I., and Smith, A. BMP induction of Id proteins suppresses differentiation and sustains embryonic stem cell self-renewal in collaboration with STAT3. Cell 115: 281-292 (2003).
- Xu, R.H., Chen, X., Li, D.S., Li, R., Addicks, G.C., Glennon, C., Zwaka, T.P., and Thompson, J.A. BMP4 initiates human embryonic stem cell differentiation to trophoblasts. Nat Biotechnol 20: 1261-1264 (2002).
Additional Publications
- Mao X; An Q; Xi H; Yang X-J; Zhang X; Yuan S; Wang J; Hu T; Liu Q; Fan G. Single-Cell RNA Sequencing of hESC-Derived 3D Retinal Organoids Reveals Novel Genes Regulating RPC Commitment in Early Human Retinogenesis. Stem Cell Rep in press :e2609 (2019).
- Schaefer SA; Higashi A; Loomis B; Schrepfer T; Wan G; Corfas G; Dressler GR; Duncan RK. From Otic Induction to Hair Cell Production: Pax2EGFP Cell Line Illuminates Key Stages of Development in Mouse Inner Ear Organoid Model. Stem Cells Development 27:237-251 (2018).
- Koehler KR; Ine J; Longworth-Mills E; Liu X-P; Lee J; Holt JR; Hashino E. Generation of inner ear organoids with functional hair cells from pluripotent stem cells. Nat Biotechnol 35:583-589 (2017).
- Noack C; Haupt LP; Zimmerman W-H; Streckfuss-Bomeke; Zelarayan LC. Generation of a KLF15 homozygous knockout human embryonic stem cell line using paired CRISPR/Cas9n, and human cardiomyocytes derivation. Stem Cell Research 23:127-131 (2017).
- Saxena S; Ronn RE; Guibentif C; Moragheba R; Woods N-B. Cyclic AMP signaling through Epac axis modulates human hemogenic endothelium and enhances hematopoeitic cell generation. Stem Cell Rep doi:10.1016/j.stemcr.2016.03.006 (2016).
- Qian X; Kim JK; Tong W; Villa-Diaz LG; Krebsbach PH. DPPA5 supports pluripotency and reprogramming by regulating NANOG turnover. Stem Cells 34:588-600 (2016).
- Qian X. "Defining the mechanism by which synthetic polymer surfaces support human pluripotent stem cell self-renewal." PhD Thesis, University of Michigan (2015).
- Zhu S; Wang H; Deng S. Reprogramming fibroblasts toward cardiomyocytes, neural stem cells and hepatocytes by cell activation and signaling-directed lineage conversion. Nature Protocols 10, 959-973 (2015).
- Villa-Diaz LG; Kim JK; Lahann J; Kresbach PH. Derivation and long-term culture of transgene-free human induced pluripotent stem cells on synthetic substrates. Stem Cells Trans Med 3:1410 (2014).
- Chiang P-M; Wong PC. Differentiation of an embryonic stem cell to hemogenic endothelium by defined factors: Essential role of bone morphogenetic protein 4. Development 138: 2833-2843 (2011).
- Wang, B., Miyagoe-Suzuki, Y., Yada, E., Ito, N., Nishiyama, T., Nakamura, M., Ono, Y., Motohashi, N., Segawa, M., Masuda, S., Takeda, S. Reprogramming Efficiency and Quality of Induced Pluripotent Stem Cells (iPSCs) Generated from Muscle-derived Fibroblasts of MDX Mice at Different Ages. PLOS Currents Muscular Dystrophy. 2011 Oct 27 . Edition 1. doi: 10.1371/currents.RRN1274 .
- Yanes O; Clark J; Wong DM; Patti GJ; Sanchez-Ruiz A; Benton HP; Trauger SA; Desponts C; Ding S; Siuzdak G. Metabolic oxidation regulates embryonic stem cell differentiation. Nature Chemical Biology 6:411 (2010).