Abstract
Brazil typifies the land use changes happening in South America, where natural vegetation is continuously converted into agriculturally used lands, such as cattle pastures and croplands. Such changes in land use are always associated with changes in the soil nutrient cycles and result in altered greenhouse gas fluxes from the soil to the atmosphere. In this study, we analyzed literature values to extract patterns of direct nitrous oxide (N2O) emissions from soils of different ecosystems in Brazil. Fluxes from natural ecosystems exhibited a wide range: whereas median annual flux rates were highest in Amazonian and Atlantic rainforests (2.42 and 0.88 kg N ha−1), emissions from cerrado soils were close to zero. The decrease in emissions from pastures with increasing time after conversion was associated with pasture degradation. We found comparatively low N2O-N fluxes from croplands (−0.07 to 4.26 kg N ha−1 yr−1 , median 0.80 kg N ha−1 yr−1) and a low response to N fertilization. Contrary to the assumptions, soil parameters, such as pH, Corg, and clay content emerged as poor predictors for N2O fluxes. This could be a result of the formation of micro-aggregates, which strongly affect the hydraulic properties of the soil, and consequently define nitrification and denitrification potentials. Since data from croplands mainly derived from areas that had been under natural cerrado vegetation before, it could explain the low emissions under agriculture. Measurements must be more frequent and regionally spread in order to enable sound national estimates.

Original content from this work may be used under the terms of the Creative Commons Attribution 3.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
The Food and Agriculture Organization of the United Nations (FAO 2014) has ranked Brazil as the third largest emitting country of greenhouse gases (GHG) from agriculture for the year 2012. One reason is the significant increase in area used for agriculture in recent years (Cederberg et al 2009). However, GHG budgeting at national scales is full of uncertainties, particularly for such large countries, and too little is known about the processes that affect such estimations. Literature reviews are one viable first step towards improvements (e.g. Jungkunst et al 2006), which can eventually lead to further extrapolations to the national scale (e.g. Brocks et al 2014). For the agricultural sector nitrous oxide (N2O) is one of the most important GHG. It is emitted from soils by natural processes, which can be enhanced and potentially reduced by anthropogenic activities, such as fertilization and land use changes. The principle underlying prerequisite for N2O emission is the availability of nitrogen. Nitrogen in soils is increased by fertilization, which usually leads to increased N2O emissions. The Intergovernmental Panel on Climate Change (IPCC 2006) assumes this relationship between N fertilization and N2O emissions to be linear and defines an emission factor (EF) of 1% (1 kg of every 100 kg of applied N fertilizer is lost as N2O-N). At the same time, the IPCC strives to improve this approximation to more detailed region-specific approaches. Based on data from temperate climates (e.g. Boeckx and van Cleemput 2001), as well as global scale data (Shcherbak et al 2014), a single emission factor is imprecise. Shcherbak et al (2014) rather proposed a nonlinear relationship at the global scale. These insights lead to the conclusion that the relationship between fertilizer input and N2O emissions must vary according to environmental settings like climate and soil conditions (Jungkunst et al 2006). Considerably less data exists for the tropics compared with temperate regions (Shcherbak 2014). However, data available from tropical areas indicate that the emission factor used by the IPCC overestimates measured fluxes (Madari et al 2007, Jantalia et al 2008, Alves et al 2010, Cruvinel et al 2011, Alvarez et al 2012, Carmo et al 2013, Carvalho et al 2013, Lessa et al 2014).
Estimations for national N2O inventories are challenging, because soils are diffusive emitters and direct N2O fluxes show extremely high temporal and spatial heterogeneities (e.g. Groffman et al 2009). The largest emissions of N2O mainly result from denitrification under hypoxic conditions (Davidson et al 2000); especially during changes between well aerated (WFPS at 40–60%) and wet (WFPS ≥ 80%) conditions (Vor et al 2003). Consequently, when soil moisture increases during wet seasons, N2O emissions commonly increase as well (e.g. Luizão et al 1989). In irrigation experiments by Vasconcelos et al (2004) and Carvalho et al (2013), N2O fluxes increased after irrigation during the dry season in Brazil.
Luizão et al (1989) and Sotta et al (2008) reported that finer textured soils have a higher N availability. Additionally, Matson et al (1990) and Sotta et al (2008) measured higher N2O losses from clay soil compared to sand soil. Since tropical soils of Brazil are commonly rich in clay and experience regular changes in moisture through seasonal rainfall patterns, Brazilian soils should emit higher amounts of N2O than temperate soils. However, reported measurements show fairly low emission levels. Stable micro-aggregates, which form due to adhesion of fine soil particles and (iron-)oxides, are known to create a coarser structure. This leads to better drainage and more oxic conditions than would be expected by measured clay contents. When compared with other predominant soils at the global scale, the general role of clay content as an indicator for N2O fluxes can be questionable.
Besides accounting for N2O emission from specific land use types, N2O dynamics during actual land use changes should be accounted for, particularly with respect to the rapid land use change happening in Brazil. The expansion of cattle ranching is suggested to be the main driver of recent deforestation in the Legal Amazon (e.g. Barona et al 2010). However, Morton et al (2006) reported an increasing trend of cropland deforestation (direct conversion of forest to cropland) between 2001 and 2004 in the state of Mato Grosso.
Tropical forests have high rates of biological turnover and decomposition. High soil moisture and N availability increase these soils emissions of N2O (Davidson et al 2000). Breuer et al (2000) estimated a N2O budget of 3.55 Tg N2O-N yr−1 from tropical rainforest soils. In contrast to rainforests, reported emissions from soils under natural cerrado vegetation (forest to treed grassland ecosystems) were usually very low (Davidson et al 2001) or even negative (e.g. Carvalho et al 2013). Consequently, knowing the natural ecosystem present before the land use change may be as important in estimating N2O emissions as knowing the current agricultural land use.
Here, we focus on the regional scale within Brazil in order to improve estimates for atmospheric N2O increase. Considering single studies without a systematic scientific compilation is neither sufficient to identify regional measurement gaps nor to identify underlying key processes. The value of understanding specific soil and management properties to indicate N2O fluxes not only lies in better approximations, but also feeds process-based models that are eventually needed for scenario calculations to derive mitigation strategies. A systematic review additionally can help to derive research strategies and to set the basis for regional and temporal N2O measurement recommendations, based on revealed relationships with environmental parameters. The improved process understanding enables better national estimations.
To provide this we used reported emissions of N2O-N from soils under different land use. Specifically, we aimed to (1) compare reported N2O-N fluxes from different land use types and define average annual emissions, (2) evaluate if specific soil and management properties can serve as an indicator of N2O fluxes, and (3) determine knowledge gaps for improvements of future national N2O inventory and process understanding.
2. Materials and methods
2.1. Data collection and calculation
We searched English literature for N2O-N flux data from soils under different land use across Brazil using online databases (Web of Science, Science Direct, Scielo (Brazilian)) and search engines (Google Scholar) between March 2014 and January 2015. Search queries initially included the keywords 'N2O' AND 'soil' AND 'Brazil', which resulted in large numbers of studies (e.g. 73 studies in the Web of Science). We further specified the search by adding the keyword 'conversion'. Additional specification of the single land use types (AND 'rainforest'/'pasture'/'cropland') or geography (AND 'Amazon'/'Southern Brazil') did not result in additional studies. According to the guidelines of Aiassa et al (2015) on how to proceed on systematic reviews, we made use of personal contacts and contacted research groups in Brazil (EMBRAPA) to improve and expand our search strategies towards Portuguese studies that might have been missed using the Scielo database and due to linguistic difficulties. No time frame was set in terms of the age of the studies—the aim was to gather a good geographic coverage of Brazil. Only tabular values were analyzed; we did not extract data from graphs. Data sets were divided into three categories: (1) data from natural landscape units (Amazon forest, Atlantic forest, and cerrado), (2) land that was converted to pastures, and (3) land under agricultural management and fertilizer application. Since the third category contained long-term (one year or more), as well as short-term experiments (weeks or months), studies within this category were again divided according to the duration of the measurements. Short-term experiments usually presented as cumulative fluxes over the specific time period rather than annual emissions. Nevertheless, we treated short-term experimental data as long-term data if authors extrapolated to annual values (e.g. Metay et al 2007). Similarly, cumulative data resulting from different crops within a rotation (e.g. corn/bean rotation, Cruvinel et al 2011) were extrapolated to annual values, if the whole rotation cycle covered one year. Reported units varied among the studies, thus we converted data sets to identical units (e.g. kg N ha−1 for N2O fluxes and fertilizer inputs, or g kg−1 for Corg). If N2O fluxes were not given in annual emissions, but mean daily values were given, reported data were projected to one year. For studies which distinguished between dry and wet seasons, the length of the specific period was used in the extrapolation. Soil types were classified according to the World Reference Base (IUSS 2014). Soil texture, usually expressed as clay content, carbon content, and pH have been shown to influence N2O-N emissions. Thus, we looked at correlations with available soil properties, as well as with the amount of applied fertilizer. The latter did not include studies including soybean because legumes are treated differently by the IPCC. Forests and cerrados were not included in the regression analysis because information on the specific soil properties were derived from the mineral soil, but not from the overlying humus layer. We differentiated between fertilized and unfertilized plots (usually pastures and croplands). Except for correlations with the amount of applied fertilizer, we only regarded fertilizer-induced emissions (N2O-N/added N). Data from pastures were ordered according to the time since establishment, as pasture ages turned out to be a meaningful factor in forest areas converted to pastures (e.g. Wick et al 2005, Neill et al 2005).
2.2. Statistical analyses
We used the linear regression method to analyze the relationships between soil properties and N2O-N emissions. The influence of the applied fertilizer was additionally adapted by a nonlinear model, following the suggestions of Shcherback et al (2014). Relationships were regarded as being statistically significant for a p value of (or smaller than) 0.05. The regression analyses and creation of graphics were conducted using the R software (version 2.15.0).
3. Results
In total, 37 study sites were analyzed based on land use, soil properties, management, and fertilization (table A1erlaa1347t5).
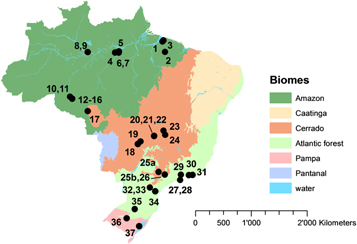
The geographical locations of the sites (figure 1) divided Brazil into regional land use types: studies conducted in the northern states (e.g. Pará, Rondônia) mainly dealt with N2O-N emissions from rainforest and cattle pastures. These land use types represented the fundamental land use change (deforestation) in the Amazon region. In contrast, studies from the central and southern states focused on conversion of cerrado area to croplands and the influence of different crop rotations, management, and fertilization strategies.
Figure 1. Sites with available N2O-N flux data in different biomes in Brazil (IBGE 2013). Numbers are according to table A1 erlaa1347t5 in the
Download figure:
Standard image High-resolution imageAnnual N2O-N emissions collected in this study (tables 1 to 3) were differentiated according to the land use type. Table 1 summarizes data from forest (Amazon and Atlantic forest), cerrado, and pasture sites. Table 2 summarizes data from experiments on cropland. Table 3 gives an overview of N2O-N fluxes from short-term experiments on grassland, pasture and cropland.
Table 1. Annual N2O emissions with minimum, median, and maximum value from forest, cerrado, and pasture soils (references are according to table A1).
| Annual N2O emissions [kg N ha−1] | ||||
|---|---|---|---|---|
| Biome | Min | Median | Max | Reference |
| Forest | 0.38 | 2.29 | 16.20 | 1, 2, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 27, 28, 31 |
| Amazon Rainforest | 0.38 | 2.42 | 16.20 | 1, 2, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14 |
| Atlantic Rainforest | 0.44 | 0.88 | 3.42 | 27, 28, 31 |
| Cerrado | −0.09 | 0.14 | 1.19 | 2, 17, 18, 19, 23, 24 |
| Pasture [age] | ||||
| ≤10 | 1.32 | 2.52 | 10.16 | 9, 10, 12, 14 |
| >10 | −0.27 | 0.90 | 3.62 | 2, 10, 11, 12, 14, 18, 19, 23, 28, 29 |
Table 2. Land use, treatment, N application, and annual N2O fluxes from soils under agricultural use in Brazil. Site numbers are according to table A1erlaa1347t5.
| Site no. | Land use | Treatment | Duration [days] | Applied N [kg N ha−1] | Annual N2O [kg N ha−1] |
|---|---|---|---|---|---|
| improved fallow plot | |||||
| 3 | cropland (agroforestry) | inga edulis | ∼365 | 0 | 0.71 |
| acacia mangium | 0 | 0.88 | |||
| control | 0 | 0.82 | |||
| conventional tillage: | |||||
| 17 | cropland | rice (1 year) | 7a | 0.85 | |
| rice (2 years) | 15 | 0.63 | |||
| 19 | cropland | crop succession | 92 | 0.57 | |
| crop-pasture rotation | 222 | 2.00 | |||
| 20 | cropland | disc harrowing (15 cm) | ∼182 | 114 | 0.04b |
| direct seeding | 114 | 0.01b | |||
| 22 | cropland | corn | 0 | 0.35 | |
| 365 | 80 | 0.54 | |||
| beans | 0 | 0.20 | |||
| 80 | 0.30 | ||||
| MF (NPK) | 60 | ||||
| MF (NK) + filter cake | 122 | ||||
| a) cropland (plant cane) | MF (NP) + vinasse | ∼365 | 87 | ||
| MF (N), + vinasse + filter cake | 149 | ||||
| MF | 120 | ||||
| 25 | b) cropland (ratoon cane) | MF + vinasse | 142 | ||
| 7 Mg trash, MF | 120 | ||||
| 7 Mg trash, MF + vinasse | 142 | ||||
| 14 Mg trash, MF | ∼242 | 120 | |||
| 14 Mg trash, MF + vinasse | 142 | ||||
| 21 Mg trash, MF | 120 | ||||
| 21 Mg trash, MF + vinasse | 142 | ||||
| 32 | cropland | tillage | 365 | 165 | 2.42 |
| no-tillage | 165 | 1.26 | |||
| 33 | pasture/cropland | integrated crop-livestock | 225 | 4.26 | |
| (corn/grazed annual-ryegrass) | 365 | (+excreta) | |||
| cropland | continuous crop | ||||
| (annual-ryegrass) | 165 | 1.26 | |||
| native vegetation | 0 | 0.65 | |||
| no tillage: | |||||
| sorghum/wheat (year 1 and 2)b | 365 | 195/253 | 0.65 | ||
| 35 | cropland | corn/wheat (year 1 and 2)c | 162/94 | 0.71 | |
| conventional tillage: | |||||
| sorghum/wheat (year 1 and 2)b | 365 | 171/253 | 0.71 | ||
| corn/wheat (year 1 and 2)c | 141/78 | 0.80 | |||
| pigeon pea + corn | 367.6 | 1.32 | |||
| lablab + corn | 167.5 | 1.12 | |||
| 37 | cropland | vetch + corn | 347 | 144.8 | 0.81 |
| black oat + corn | 98.3 | −0.07 | |||
| black oat + vetch/corn + cow pea | 231.7 | 1.32 | |||
| median | 0.80 |
MF: Mineral fertilizer aFrom Carvalho et al (2007). bReported as annual emissions. cSoybean/vetch (year 1), sorghum/wheat (year 2). dMaize/wheat (year 1), soybean/vetch (year 2).
Table 3. Land use, treatment, N application, and cumulative N2O fluxes from soils under agricultural use in Brazil. Site numbers are according to table 5.
| Site no. | Land use | Treatment | Duration [days] | Applied N [kg N ha−1] | Cum. N2O [kg N ha−1] |
|---|---|---|---|---|---|
| control | 0 | 0.07 | |||
| 15 | pasture | tillage | 42 | 0.23 | |
| no-till, co-planting rice | ∼182 | 33 | 1.10 | ||
| no-till, co-planting soybean | 0 | 1.10 | |||
| control | 0 | 0.07 | |||
| 16 | pasture | tillage | 180 | 42 | 2.23 |
| no-tillage | 33 | 1.62 | |||
| urine application | 94/37 | 396a/683b | |||
| 21 | pasture | dung application | 94/37 | 188a/346b | |
| corn | 173 | 155.3 | 0.20 | ||
| 24 | cropland | irrigated bean | 135 | 102.7 | 0.20 |
| soybean | 153 | 21.2 | 0.10 | ||
| cotton | 258 | 0 | 0.10 | ||
| 26 | grassland | control | 0 | 0.02 | |
| urine application | 30 | 860 | 1.69 | ||
| vegetation cycles | |||||
| 30 | grassland | V1 | 162 | 80 | 0.44 |
| V2 | 178 | 100 | 0.57 | ||
| V3 | 149 | 80 | 1.47 | ||
| control | 0 | 0.04 | |||
| 34 | pasture | urine application | 90 | 2200 | 5.87 |
| dung application | 1110 | 1.43 | |||
| fertilization with pig slurry | |||||
| 36 | cropland | no-tillage | 28 | 191 | 0.40 |
| conventional tillage | 191 | 0.51 |
3.1. Soil under natural vegetation
In general, N2O emissions from forest soils were higher than emissions from pasture sites (e.g. Verchot et al 1999, Steudler et al 2002, Wick et al 2005, Carmo et al 2012). Reported N2O-N fluxes from forest soils were positive without exception, but varied from 0.38 up to 16.20 kg N ha−1 yr−1. Rainforests (Amazonas and Atlantic rainforest) differ considerably from cerrado: the highest emission (16.20 kg N2O-N ha−1 yr−1) was reported from a forest site in the Amazon, whereas the maximum emission from Atlantic forest was much lower (3.42 kg N2O-N ha−1 yr−1). In contrast, emissions from cerrado sites were exceptionally low (median: 0.14 N2O-N ha−1 yr−1 with a maximum of 1.19 N2O-N ha−1 yr−1), and often below the detection limit (<0.6 ng cm−2 h−1).
Data presented in table 1 includes studies conducted in primary or moderately altered forests. Studies from Verchot et al (1999), Vasconcelos et al (2004), and Coutinho et al (2010) present data from secondary forests of 12, 20, and 34 years after reforestation. Here, annual N2O-N emissions amount to 0.35, 0.94, and 0.88 kg ha−1, respectively. These results are lower than the median annual emission from primary forests reported in this study and are in the range of annual emissions from pastures.
3.2. Pasture soil
N2O-N fluxes from pasture soils varied widely, too, but emissions from pastures younger than 10 years were significantly (p < 0.05) higher than from older pastures (table 1, figure 2). Thus, we differentiated data from pastures of 10 years and younger from those older than 10 years. Neill et al (2005) modeled the behavior of annual N2O emissions from forests and pasture sites of different ages as an exponential function. When we fitted an exponential function to our data ( with y = flux of N2O-N [kg ha−1 yr−1] and
with y = flux of N2O-N [kg ha−1 yr−1] and  age), we found a similar decrease in N2O-N emissions with pasture age (figure 2).
age), we found a similar decrease in N2O-N emissions with pasture age (figure 2).
Figure 2. An exponential model of annual N2O-N flux data (n = 24) dependent on years after conversion (R2 = 0.48). Pasture ages varied from 1 to 87 years. The gray shaded area represents the 95% confidence interval. The broken line is the fit of Neill et al (2005), based on N2O-N emissions from pastures of different ages (n = 9) in the state of Rondônia ( In their study, pasture ages varied from 1 to 41 years. a) Including data from Neill et al (2005).
In their study, pasture ages varied from 1 to 41 years. a) Including data from Neill et al (2005).
Download figure:
Standard image High-resolution imageFor short-term experiments on pastures under additional fertilization and soil management (table 3), highest emissions were reported from pastures under urine application and tillage management (5.87 and 2.23 kg N ha−1). These studies were not included in the nonlinear regression (figure 2).
3.3. Cropland soil
Highest annual emissions occurred from crop-pasture rotations (4.26 kg N ha−1 yr−1) and cropland under tillage treatment (2.42 kg N ha−1 yr−1). The overall median from cropland soils was 0.80 kg N ha−1 (table 2).
We could use only 6 studies (15, 16, 22, 26, 34, and 36 in table A1erlaa1347t5) for calculating an emission factor (EF), because these allowed for subtraction of background emissions. Of these, only study 22 (Santos et al 2008) presented long-term measurements focusing on corn and bean cultivation. EFs were 0.24% for corn and 0.13% for bean. EFs from short-term experiments (table 3) ranged from 0.13 to 5.14%, with a median of 0.38%.
Except for pH (R2 = 0.21, p = 0.06), correlations with N2O-N/added N were significant (figures 3(a)–(c) and table 4), but not important. Clay contents covered a range from 13 to 86%, and the linear regression implied only a slightly increasing trend for fertilized plots (0.0009) and a slightly decreasing trend (−0.003) for unfertilized plots with higher clay contents. N2O-N/added N slightly decreased with increasing pH (−0.03) or carbon content (−0.06). On unfertilized plots, emissions increased with increasing pH (0.41), but emissions decreased with increasing carbon content (−1.38).
Figure 3. Relationships between N2O-N fluxes [kg ha−1 yr−1] per added N [kg N ha−1 yr−1] and (a) soil clay content [%], (b) pH, (c) carbon content [%], and relationship between N2O-N fluxes and (d) the annual amount of applied fertilizer N [kg ha−1 yr−1]. Figure 3 (d) also includes the model of Shcherbak et al (2014) (excluding N-fixing crops) (broken line). Only fertilized plots (croplands and pastures) are presented. Gray shaded areas represent the 95% confidence intervals; see table 4 for more precise information on the regressions. * = p-value < 0.05.
Download figure:
Standard image High-resolution imageTable 4. Intercepts and slopes (including the lower and upper values of 95% confidence interval) for linear regression between soil properties and N2O-N/added N ratio for fertilized and unfertilized plots, and applied N and N2O-N fluxes for the linear and nonlinear regression. * = p-value < 0.05.
| Clay content | pH | Corg | Applied N | |||||
|---|---|---|---|---|---|---|---|---|
| Regression parameter | fertilized | unfertilized | fertilized | unfertilized | fertilized | unfertilized | linear | nonlinear |
| intercept | −0.22 | 1.68 | 0.16 | −1.29 | 0.18 | 3.84 | 0.544 | 0.93 |
| slope | 0.000 09* | −0.003 | −0.03 | 0.41* | −0.06* | −1.38 | 0.004 | 1.98* |
| lower | 0.0002 | −0.017 | −0.06 | 0.07 | −0.10 | −9.04 | 0.0009 | 0.43 |
| upper | 0.0016 | 0.010 | 0.002 | 0.74 | −0.02 | 6.28 | 0.0068 | 3.54 |
| slope | −0.15 | |||||||
| lower | −1.71 | |||||||
| upper | 1.41 | |||||||
| R2 | 0.29* | 0.007 | 0.21 | 0.51* | 0.57* | 0.23 | 0.20* | 0.20* |
N2O-N fluxes increased with applied N fertilizer (figure 3(d)). In their global meta-analysis, Shcherbak et al (2014) found that a nonlinear model better described the relationship between N2O fluxes and fertilization than a linear model. In contrast, we found that a nonlinear model of the Brazilian data did not result in a better description of the relationship between emissions and fertilization compared with a linear model (R2 = 0.20* for both linear and nonlinear model). Our nonlinear model ( with y = N2O-N flux [kg ha−1 yr−1] and x = applied fertilizer [kg N ha−1]) does not compare well with that of Shcherbak et al (2014) (figure 3(d)). Besides the different intercept, which is caused by reported N2O-N fluxes at low and even zero fertilization, our model has a lower slope, due to the low emissions. Table 4 shows more detailed results of the regressions.
with y = N2O-N flux [kg ha−1 yr−1] and x = applied fertilizer [kg N ha−1]) does not compare well with that of Shcherbak et al (2014) (figure 3(d)). Besides the different intercept, which is caused by reported N2O-N fluxes at low and even zero fertilization, our model has a lower slope, due to the low emissions. Table 4 shows more detailed results of the regressions.
4. Discussion
4.1. Importance of land use
4.1.1. Natural vegetation
The different emissions from cerrado and rainforest reveal the high variability of natural N2O fluxes. While emissions from Brazilian rainforest sites were generally high (2.29 kg N2O-N ha−1), but still within the range of emissions reported for temperate (−0.1 to 4.9 N2O-N ha−1 yr−1, Jungkunst et al 2004) and Australian tropical forests (1.15 to 5.36 N2O-N ha−1 yr−1, Breuer et al 2000), N2O fluxes from cerrado soils were often close to zero, below detection limits (e.g. Pinto et al 2002, Varella et al 2004) or negative (Verchot et al 1999). Nitrification is a more important source of N2O emissions in cerrado soils because of the better drainage caused by the coarse soil structure (Pinto et al 2002). Consequently, these soils become increasingly important in terms of nitric oxide (NO) emissions. Pinto et al (2002) assumed low nitrification rates and low NO3- contents resulted in low N2O fluxes. However, further attention should be paid to cerrado soils, in order to identify the underlying processes.
The Amazonian forest soils in the north of Brazil showed higher emissions than those of the coastal Atlantic forests in the south-east of the country. Only 3 studies dealt with the Atlantic forest, but 13 were found for the Amazonian rainforest. More studies from the Atlantic forest would help to confirm this difference between the two forest types. Emissions from secondary forests (12, 20, and 34 years after reforestation) ranged between 0.35 and 0.94 kg N2O-N ha−1 yr−1 and were lower than from primary forests. This suggests that N cycles in these reforested areas had not completely recovered. Regardless of the high emissions from rainforest soils compared with soils under other land use, precise knowledge concerning emissions during the conversion from rainforest to pasture is missing. This is a key aspect, since some studies report increased N2O emissions from soil after conversion of forest to pasture (Keller et al 1993, Veldkamp et al 1999, Davidson et al 2001, Melillo et al 2001). They explain this event with a temporal increase of N availability. The removal of plants as a sink for nutrients causes very high nutrient availability in soils (Bormann and Likens 1979), which is known to increase N2O emissions at barren sites (Repo et al 2009). In addition to the emissions from soil to atmosphere, soil–water degassing can be an important source for N2O fluxes directly after forest clear-cutting (Bowden and Bormann 1986).
4.1.2. Pastures
According to Keller and Reiners (1994) and Melillo et al (2001), young pastures have increased emissions directly after a clear-cut, followed by decreasing emissions as the pastures age. Decreasing denitrification rates (N2O + N2) in mid-successional sites compared with primary forest and early successional sites may explain this trend (Robertson and Tiedje 1988).
The duration of higher N2O emissions after the creation of a pasture varies from 3 months (Elligson et al 2000), to over 2 years (Melillo et al 2001), to up to 10 years (Keller et al 1993). In our review, we found that N2O emissions from young pastures (≤10 years) were significantly higher than from older pastures. According to Davidson et al (2001), Brachiaria spp. grasses, which were introduced from Africa (Boddey et al 2004) and are commonly used for pastures in Amazonia, can be effective sinks for soil N. Quick immobilization of nitrogen that is released after the disturbance of the soil might delay the degradation of the pasture. Subbarao et al (2009) found a reduction in N2O emissions of more than 90% under plots with Brachiaria species compared with soybean plots. The Brachiaria roots produce and deliver nitrification inhibitors to soil-nitrifier sites (Subbarao et al 2009). In grazed Brachiaria pastures, intense uptake of nitrogen by grazing animals degrades pastures (Boddey et al 2004). The decrease of available N in the litter leads to a reduction in the amount of N available for plant growth. Cerri et al (2005) and Hohnwald et al (2006) also report that many pastures suffer from degradation (declining fertility and grass productivity, and increasing weed cover) already 4 to 10 years after establishment. Thus, pastures are unsustainable—a point supported by our finding of decreasing N2O fluxes from pastures about 10 years after conversion.
4.1.3. Croplands
Except for one study (site no. 3), data from croplands were derived from areas that had been under cerrado vegetation before. This might justify the low emissions from cropland, since cerrado soils appear to be a less considerable source for N2O fluxes. Although N fertilization increased emissions for short periods of 3 to 7 days after application, the reaction of the soil to N addition at the annual scale was very low. For application rates below 100 kg N ha−1, which are frequently applied, the reaction was negligible. The data collected in this study did not fully agree with the global nonlinear model suggested by Shcherbak et al (2014). Their model includes data from 84 locations worldwide, and is consequently designed for a much larger scale than our country-specific analysis. This difference emphasizes that large scale or global relationships may be inappropriate to apply to more regional aspects.
Annual fluxes of N2O-N from cropland soils in Brazil ranged from −0.07 to 4.26 kg N ha−1, with a median of 0.80 kg N ha−1 (table 2). This value is much lower than emissions reported by Roelandt et al (2005) from croplands in Canada (2.27 kg N2O-N ha−1 yr−1), Europe (2.47 kg N2O-N ha−1 yr−1), and the United States (3.35 kg N2O-N ha−1 yr−1). Highest emissions (figure 3(d)) occurred from the two cropland areas that were under either conventional tillage (2.42 kg N2O-N ha−1 yr−1; Piva et al 2012) or integrated cropping systems (4.26 kg N2O-N ha−1 yr−1; Piva et al 2014).
4.2. Importance of soils
4.2.1. Soil texture and structure
Soil texture and structure are highly relevant driving factors for N2O emissions, mainly as controllers of water balances and nutrient availability. Generally, finer textured soils have a higher N availability (Luizão et al 1989) and consequently emit higher amounts of N2O than sandy soils (Matson et al 1990). In a laboratory experiment, N losses from heavily weathered tropical soils were higher in a clay textured soil variation than from a sandy variation (Sotta et al 2008). Based on these findings Sotta et al (2008) suggest a higher N availability in a clay compared with a sand soil in Amazonian forests. Due to the good drainage of sandy soils, anaerobic conditions are rare and the potential for denitrification is low. In this study, clay proved to be a poor predictor for N2O emissions from fertilized (slope = 0.0009) and unfertilized plots (slope = −0.003), most likely due to the formation of micro-aggregates and the associated different water retention properties. Tomasella et al (2000) mention the rapid decrease in water content between saturation and −100 kPa, and underline that Brazilian soils behave more like coarse-textured soils. As a result, the water holding capacity does not necessarily increase with increasing clay content, and nitrification is more likely to occur than denitrification. Therefore, tropical soils with high clay contents, formation of micro-aggregates, and high drainage can be expected to emit less N2O than is reported for temperate soils. Thus, the clay content is not necessarily a reliable indicator for N2O-N emissions from Brazilian soils.
4.2.2. Soil chemical properties
In this study, neither pH nor Corg content seemed to have an influence on N2O-N fluxes. However, fertilized and unfertilized plots differed. On fertilized plots, linear regression slopes were negative for both pH (−0.03) and Corg content (−0.06). For unfertilized plots, pH (slope = 0.41) turned out to be more predictive than Corg (slope = −1.38). Although both parameters have been reported to influence denitrification rates (Knowles 1982), the general findings within this study suggest that N2O fluxes occur from nitrification. Thus, pH and Corg are of secondary importance. The contribution of pH and especially Corg in the formation of micro-aggregates, however, should be further investigated.
4.3. Knowledge gaps
Considerable data gaps exist for certain biomes. We found no reported N2O emissions from the Caatinga and Pantanal biomes. Except for one site, data from croplands were derived from areas that had been established in cerrado areas, which were found to have extremely low emitting soils under natural vegetation. This lack of data hinders our ability to explain the low emissions from croplands, even after fertilizer application, and points out the need for measurements from additional land use types.
Since N2O emissions exhibit short-termed emission peaks caused by environmental changes, high temporally resolved measurements are needed in order to explain mechanisms. Automated measurements enable continuous data acquisition, but the establishment of such studies is restricted to sites with a power supply and, for certain approaches, flat topography. Therefore, to achieve an adequate spatial measurement distribution across a large nation such as Brazil, we still have to rely on manual measurements that also take into consideration environmental (dry/wet cycles) and human induced (land conversion) changes. Biweekly measurements throughout the year, as done by most authors, are no longer suitable for increasing our understanding of biogeochemical processes. Furthermore, exact knowledge of how N2O-N emissions change during land conversion is missing and desperately needed, since this time frame may likely account for large emission pulses that need to be accounted for in national budgeting.
Improving the existing understanding of the underlying processes, especially during land conversion can only be ensured by consistent monitoring and frequent measurements. Such monitoring data could provide the basis for further model refinement and allow for spatial and temporal extrapolations. The goal would be to develop regional solutions to improve national inventories. At this point, most process-oriented models have been developed for temperate conditions and application to tropical conditions is challenging. The different hydraulic conditions caused by micro-aggregates need to be considered, since adequate description of the soil moisture is a prerequisite for modelling N2O-N fluxes from soils.
5. Conclusions
This systematic review on N2O fluxes from Brazilian soils provides a good basis for further estimations and inventories on the national scale and eventually for explaining atmospheric N2O increases. The land use types differed in direct N2O fluxes from soils, but emissions were generally low. Systematic regional measurement gaps were identified, of which the Caatinga biome in northeastern Brazil is the most prominent example. Furthermore, land use types were not randomly distributed between biomes. In other words, pastures were studied in rainforest biomes, and croplands in cerrado biomes. Therefore, no predictions can be made on the behavior of N2O fluxes from croplands in the rainforest biome. Soil parameters, such as pH, Corg, and clay content, had proven to be unsuitable as indicators for N2O fluxes. Oddly enough, N2O itself was found to be an indicator for the degradation stage of pastures, as emission levels decrease along with the productivity and years since conversion. A kind of tipping point from high to low N2O emissions was found to be in the range of 10–15 years after forest conversion. N2O is known to have high event-based emissions, which the current measurement concept did not account for. Future studies must focus on high temporal resolutions in order to promote process understanding. Otherwise, sound national inventories will not be possible.
Acknowledgments
The authors would like to thank the BMBF (Federal Ministry of Education and Research) for financial support for the project Carbiocial and the anonymous reviewers for their helpful comments. We also thank Dr Thomas Horvath, who truly helped to improve our manuscript.
Appendix
Table A1. Geographical location, climatic condition, soil type and properties of studies with N2O-N flux measurements in Brazil.
| Geographical location [°] | ||||||||
|---|---|---|---|---|---|---|---|---|
| Site no. | Reference | south | west | MAT [°C] | MAP [mm] | Soil type WRB | Clay content [%] | Corg [g kg−1] |
| 1 | Vasconcelos et al (2004) | 1.31 | 47.95 | 25.5 | 2539 | Ferralsol | 20 | 2.20 |
| 2 | Verchot et al (1999) | 2.98 | 47.51 | 1850 | Ferralsol | 2.47–3.02 | ||
| 3 | Verchot et al (2008) | 1.11 | 47.78 | 26 | 2500 | Leptosol | ||
| 4 | Keller et al (2005) | 3.03 | 54.95 | 25 | 2000 | Ferralsol | 80 | 2.50 |
| Acrisol | 38 | |||||||
| 5 | Davidson et al (2004) | 2.88 | 54.95 | 2000 | Ferralsol | 60–80 | ||
| 6 | Silver et al (2005) | 2.64 | 54.59 | 25 | 2000 | Ferralsol | 60 | |
| Acrisol | 18 | |||||||
| 7 | Varner et al (2003) | 2.64 | 54.59 | 25 | 2000 | Ferralsol | 80 | |
| Acrisol | 38 | |||||||
| 8 | Livingston et al (1988) | 3.0 | 60.0 | 26 | 1770 | Ferralsol | ||
| Acrisol | ||||||||
| 9 | Luizão et al (1989) | 3.0 | 60.0 | 2200 | Ferralsol | |||
| 10 | Melillo et al (2001) | 10.16 | 62.81 | 25.6 | 2200 | Acrisol | 19–29 | |
| 11 | Steudler et al (2002) | 10.16 | 62.81 | 25.5 | 2200 | Nitisol | 23–29 | |
| 12 | Garcia-Montiel et al (2001) | 10.5 | 62.5 | 18.8–25.6 | 2270 | Acrisol | <30 | |
| 13 | Garcia-Montiel et al (2003) | 10.5 | 62.5 | 18.8–25.6 | 2270 | Acrisol | 20–30 | |
| 14 | Neill et al (2005) | 10.5 | 62.5 | 25.6 | 2200 | Acrisol | 13–76 | |
| 15 | Carmo et al (2005) | 10.5 | 62.5 | 2270 | Ferralsol | |||
| 16 | Passianoto et al (2003) | 10.5 | 62.5 | 25.5 | 2200 | Acrisol | ||
| 17 | Carvalho et al (2009) | 12.48 | 60.0 | 23.1 | 2170 | Ferralsol | 73 | 1.71–2.77 |
| 18 | Neto et al (2011) | 17.78 | 51.91 | 23.3 | 1550 | Ferralsol | 54–68 | 1.85–2.90 |
| 19 | Carvalho et al (2014) | 17.36 | 51.48 | 23 | 1500–1800 | Ferralsol | 56–60 | 2.09–2.89 |
| 20 | Metay et al (2007) | 16.48 | 49.28 | 22.5 | 1500 | Ferralsol | 40 | |
| 21 | Lessa et al (2014) | 16.48 | 49.28 | Ferralsol | 43 | |||
| 22 | Santos et al (2008) | 16.48 | 49.28 | 22.5 | 1460 | Ferralsol | ||
| 23 | Varella et al (2004) | 15.65 | 47.75 | 1500 | Ferralsol | 57–74 | 2.41 | |
| 4.74 | ||||||||
| 24 | Cruvinel et al (2011) | 16.3 | 47.5 | Ferralsol | 49–72 | |||
| 16.25 | 47.61 | Ferralsol | 68–76 | |||||
| 25a | ||||||||
| 25b | Carmo et al (2013) | 22.25 | 48.56 | 21 | 1390 | Lixisol | 11 | |
| 22.68 | 48.55 | Ferralsol | 29 | |||||
| 26 | Barneze et al (2014) | 22.7 | 47.61 | Nitisol | 3.03 | |||
| 27 | Neto et al (2011) | 23.56 | 45.08 | 3050 | 35 | 4.59–9.15 | ||
| 23.4 | 45.18 | 2300 | 21 | |||||
| 28 | Carmo et al (2012) | 23.31 | 45.08 | 19.1–25.5 | 2500 | Acrisol | 23 | |
| 23.4 | 45.25 | |||||||
| 29 | Coutinho et al (2010) | 22.73 | 44.95 | 20 | 1500 | Ferralsol | 30–36 | |
| 30 | Morais et al (2013) | 22.76 | 43.68 | 24 | 1300 | Acrisol | 45 | |
| 31 | Maddock et al (2001) | 22.75a | 43.08a | 21 | 1500–2250 | Ferralsol | 27–29 | 2.01 |
| 2.03 | ||||||||
| 32 | Piva et al (2012) | 24.78 | 49.95 | 1400 | Ferralsol | 44 | 2.87 | |
| 3.05 | ||||||||
| 33 | Piva et al (2014) | 24.78 | 49.95 | 1400 | Ferralsol | 44 | 2.87 | |
| 2.93 | ||||||||
| 34 | Sordi et al (2014) | 25.38 | 49.11 | 1400 | Cambisol | 44 | 2.50 | |
| 35 | Jantalia et al (2008) | 28.25 | 52.4 | 1430 | Ferralsol | 63 | ||
| 36 | Giacomini et al (2006) | 29.75 | 53.7 | Luvisol | ||||
| 37 | Gomes et al (2009) | 30.1 | 51.7 | 19.4 | 1440 | Acrisol | 22 | |
MAT: mean annual temperature. MAP: mean annual precipitation. aCoordinates from MMA/IBAMA (2006).