Abstract
The problem of reducing the impacts of rising anthropogenic greenhouse gas on warming temperatures has led to the proposal of using stratospheric aerosols to reflect some of the incoming solar radiation back to space. The deliberate injection of sulfur into the stratosphere to form stratospheric sulfate aerosols, emulating volcanoes, will result in sulfate deposition to the surface. We consider here an extreme sulfate geoengineering scenario necessary to maintain temperatures at 2020 levels while greenhouse gas emissions continue to grow unabated. We show that the amount of stratospheric sulfate needed could be globally balanced by the predicted decrease in tropospheric anthropogenic SO2 emissions, but the spatial distribution would move from industrialized regions to pristine areas. We show how these changes would affect ecosystems differently depending on present day observations of soil pH, which we use to infer the potential for acid-induced aluminum toxicity across the planet.
1. Introduction
Stratospheric sulfate injections (SSI) have been proposed [1, 2] as a possible method of temporarily offsetting some of the increase in surface temperatures produced by the anthropogenic increase of greenhouse gases (GHGs). As in the case of explosive volcanic eruptions [3], the injected SO2 quickly oxidizes, producing sulfate aerosols that reflects part of the incoming solar radiation back to space. The optically active cloud of sulfate aerosol would also spread over different latitudes due to the stratospheric circulation and reside in the much more stable stratosphere for a longer period of time (more than 12 months [4]) than tropospheric sulfate compounds which are inadvertently emitted today in combustion processes (from 3 d to a month [5, 6]). For this reason, the overall radiative effect of the sulfate particles injected in the stratosphere would be much greater than the one produced by the same amount of tropospheric sulfate [6].
Understanding the possible side effects of SSI is important to characterize before considering deployment. Many studies have used model simulations to examine possible consequences of reduced solar radiation as a proxy for the increased albedo by the injected aerosols, analyzing for instance the surface response of vegetation productivity [7] or precipitation [8]. Those analyses do not however consider the effects of the increased stratospheric sulfate burden, particularly the eventual deposition of the injected sulfate at the land surface.
The impacts of SSI strongly depend on the scenario for both the background emission pathway and the desired climate effect of the injection. Previously, some studies looking at deposition under SSI have focused on injection strategies with a fixed injection located at the equator of limited magnitude [4, 9]. Here, we show how sulfate deposition would change under a scenario where the injection amount changes each year in order to keep global surface temperature and interhemispheric and equator-to-pole temperature gradient at 2020 levels, by injecting sulfate at 4 different locations. The background emission scenario considered is RCP8.5 (a high-end scenario with unabated emissions) under which temperatures would rise by the end of the century by more than 5 °C [10, 11]. Compared to previous fixed-magnitude injection scenarios that did not have precise temperature target, the quantity of SO2 necessary here would be almost one order of magnitude larger. We use here a more complex injection strategy with complex temperature targets and a state-of-the-art model with realistic stratospheric aerosols and better resolved stratospheric circulation [12] (the Community Earth System Model version 1 with the Whole Atmosphere Community Climate Model as its atmospheric component—CESM1(WACCM) [12, 13]). This, combined with the possibility of scaling the results for more moderate emission scenarios [14] can produce a more useful scenario of SSI deployment under which it to determine the possible side effects. We focus here on the changes in global and regionally distributed S-deposition and consider how SSI might affect different parts of the world in terms of both soil acidification and population density.
2. Global deposition changes
Sulfate compounds are already produced in a variety of ways at the Earth's surface: some are natural, such as oceanic-produced dimethyl sulfide (DMS) [15] and sulfate emissions from volcanic activity [3, 16], while others are produced inadvertently by human activities, particularly through fossil fuel combustion (especially coal). Reducing this latter kind of SO2 emission has been a priority of many governments and international bodies in the last decades as it represents an air quality and acid rain problem. Global anthropogenic sulfur emissions have gone down by around 20% since they peaked in the 1980s, largely through reductions in North America and Europe [17–19].
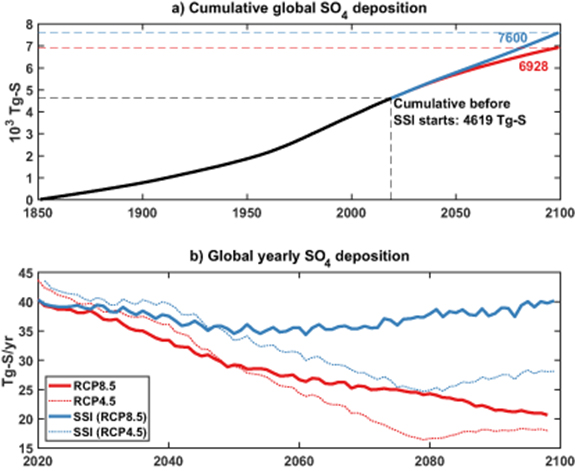
Global deposition of S is projected to decrease over the 21st century, in absence of SSI (figure 1), in all IPCC scenarios [6]. Even under the highest emission scenario, RCP8.5, global SO2 emissions are projected to decrease by more than 75% relative to 2020 [6], and therefore total deposition of sulfate would fall accordingly. In the model used in this work, over 90% of the deposition would be in the form of wet deposition (involving in-cloud and below-cloud scavenging processes) and the rest would be in the form of dry deposition (involving gravitational settling, dispersion and transportation by turbulence). In 2020, total global deposition is projected to be 40 Tg-S yr−1. By the last decade of the century, under RCP8.5 total S-deposition should decrease to 23 Tg-S yr−1. Past projections have shown that the overall clear-sky radiative forcing resulting from the decrease in tropospheric sulfate emissions would be +0.52 W m−2 by 2090–2100 [6]. In contrast, the negative forcing produced by the stratospheric aerosols in this geoengineering simulation by 2090–2100 would be −7.9 W m−2.
Figure 1. (a) Cumulated global deposition of SO4 (expressed in Tg-S) from 1850 (black line) until 2010, and for RCP8.5 (red line) and SSI (blue line, starting in 2020). (b) Yearly and globally averaged SO4 deposition (in Tg-S yr−1) in RCP8.5 (red) and RCP8.5 + SSI (blue) for the total deposition. Dotted lines of the same color show the same quantities scaled for an RCP4.5 scenario without and with SSI.
Download figure:
Standard image High-resolution imageUnder the SSI Geoengineering scenario considered here, the amount of SO2 injected in the stratosphere must increase almost linearly in order to maintain temperatures at 2020 levels to counterbalance the increased greenhouse gas forcing with RCP8.5 [11]. When this increased stratospheric injection is combined with the reduction in industrial SO2 emissions assumed under RCP8.5, the net result is that yearly global SO4 deposition is projected to be very similar between 2020 and 2100 in this particular SSI scenario. However, SSI would alter both S particle size and the spatial distribution of S deposition across the earth. SSI impacts on S-deposition is highly scenario depended: for instance, the RCP4.5 emission scenario has larger reductions in industrial S and CO2 emissions compared to RCP8.5, and thus requires less SSI geoengineering to maintain current mean temperatures [14]. In this interpolated case (figure 1(b)), by the end of the century under the SSI scenario global deposition would be only 62% of the global deposition in 2020.
3. Geographical distribution of deposition
On average, sulfur particles emitted close to the surface have a lifetime of 3–4 d [17]. For this reason, most of the S deposition is normally localized geographically close to the source of the emission [20], nonetheless a portion is transported to areas considered pristine [21, 22] (figure 2(a)). A large part of present-day sulfate emission is due to human activities, and the resulting deposition is therefore centered overpopulated areas in China, India, and near tropical urban areas, and reduced over the oceans (figure 2(a)). In figure 2(b) we show the cumulative deposition over the period of 1850–2010 to highlight that in the past there used to be much higher emissions in Europe and North America.
Figure 2. (a) Geographical distribution of SO4 deposition fluxes (g-S m−2 yr−1) for 2010–2020. (b) Cumulated SO4 deposition from 1850–2010 on a logarithmic scale (g-S m−2) (c) differences in 2080–2099 deposition between the SSI scenario and RCP8.5 (g-S m−2 yr−1). (d) Years needed in this SSI scenario to match the cumulative deposition in the historical period. For years greater than 2100, an emulator has been used to estimate the increase in temperatures and the sulfate needed (see methods).
Download figure:
Standard image High-resolution imageWhen aerosols are injected into the stratosphere, the latitudinal distribution of the surface deposition is mostly driven by stratospheric circulation and cross-tropopause fluxes, resulting in most of the aerosols being transported into the troposphere to the mid- and high- latitudes [5], as shown in figure 2(c) (mostly independent to the particular choice of injection locations, see figure S1 (available online at stacks.iop.org/ERL/15/094063/mmedia) for the deposition in a case with equatorial injection [23]).
Once the aerosols have crossed the tropopause, the precise geographical distribution of the surface deposition depends on tropospheric circulation and precipitation patterns, because wet deposition removes a large portion of the sulfate particles. For this reason, the S deposition patterns in figure 2(c) show large increases in S deposition where both atmospheric concentration and precipitation are high, for instance in the Himalayan region. Furthermore, since temperatures would rise slightly more in the northern hemisphere in this model, more SO2 is required there to maintain the inter-hemispheric temperature gradient [10], thus overall deposition is higher there.
SSI produces almost no increase in sulfate deposition between 30 N and 30 S, since the stratospheric circulation transports the aerosols from those latitudes first upwards and then at higher latitudes, where they can cross the tropopause and then quickly reach the surface [5]. In figure 2(d) we show that, for this reason, it would take more than 200 years of SSI to reach the same cumulative deposition as the historical period in many areas, while in others 20 years of SSI are enough to match it. This can happen either because anthropogenic deposition is still projected to be high in some areas in the near future (such as over parts of Asia and Africa) or because sulfate deposition from SSI is larger than what was experienced in the past (such as over the Arctic).
4. Changes in deposition patterns for populations and soils
The overall spatial pattern of deposition from SSI is not sufficient to determine its impacts on the human population and the ecosystems.
In present day conditions, the surface concentration of PM2.5 from sulfate increases with population density (figure 3(a)), due to the close proximity of most emitting sources to populated areas, and it is well known how these particles negatively impact the population [24, 25].For this reason, the strong projected decrease in anthropogenic emissions by the end of the century implies a more than halving of sulfate-related PM2.5 (SPM2.5). Even under the large amount of SSI needed by the end of the century, however, the SPM2.5 concentration would be only slightly higher than the one under RCP8.5. This is both caused by the changes in the overall deposition of sulfate shown above, and by the fact that the particles produced by SSI in the stratosphere are much larger than 2.5 microns [4, 10].
Figure 3. (a) PM2.5 (μg m−3) from sulfate concentration averaged over areas with similar population density. (b) Same as a), but with averaged temperature changes (K) compared to present day. Projected population data taken from the SSP5 scenario, 2020 for present day and 2090 for end of century.
Download figure:
Standard image High-resolution imageFurthermore, these effects on the SPM2.5 concentration should be considered in context of SSI capacity to counter greenhouse-gas driven warming (figure 3(b)). Without SSI, under RCP8.5 all population densities would incur at least 4 °C of warming, in the most populated areas and up to more than 5 °C in less populated areas. This non-uniformity in the averaged temperatures can be explained considering that most populated areas are located in zones with a temperate climate.
The problem of acid rain in the 1980s/1990s highlighted the adverse impact of sulfur deposition in some ecosystems. Our projections show that SSI would have a rather mild impact over highly populated region, due to the preeminence of S-emissions from anthropogenic sources near the ground. Here we consider the effects of S deposition due to SSI by assessing the potential of different soils to mobilize dissolved aluminum (Al) in response to acid addition. The addition of sulfate might have other effects, such as a local direct modification of the soil pH, but here we focus on dissolved Al, considering its toxicity both for aquatic ecosystems and plant nutrition [26–28], but also considering its damaging effects on water quality due to drainage from soils [27].
The potential for Al release varies with soil pH [26, 28]. Soils with an alkaline pH (pH > 7) have a low potential for mobilizing Al because Al solubility is minimal in this pH range and the carbonate and silicate minerals common in alkaline soils readily neutralize acidity [26]. Below pH 6 Al-hydroxides and Al-organic complexes neutralize acidity, the solubility of Al increases, and soil exchange sites become dominated by Al ions [26]. In soils where exchange sites are dominated by Al, acid deposition drives Al export; whereas in soils with small or intermediate concentrations of exchangeable Al, export of exchangeable base cations can temporarily buffer pH and prevent Al release [28]. Thus, to a first order, we can assume the potential for acid deposition to drive Al export is approximately linearly related to the soil's aluminum saturation percentage (ASP), with a maximum at ASP = 100%. This is consistent with critical loading studies that have assumed a linear relationship between vulnerability and soil base saturation [27].
We derived ASP from ground observations by using a large database of soil pH measurements [29] to model ASP from pH (see Methods). This approach allowed us to map the relative vulnerability of soils to Al release given observed modern soil pH and modelled S deposition rates. We combined these data using a normalized vulnerability index, where vulnerability = (ASP, %)*(S deposition rate, gS m−2 yr−1) divided by its maximum value. The vulnerability index provides an index to how much ecosystems could be harmed by acidification of the soils, through the mobilization of Al, a toxic chemical for many terrestrial and aquatic ecosystems [26, 27].
We compare acid deposition in the present day (figure 4, first panel) and at the end of century with geoengineering (second panel). Global-scale geographic patterns are broadly similar between the two scenarios because they are partly defined by the present-day distribution of acid soils, which are most common in humid climates. There are however local shifts in S deposition that affect soils differently in the two scenarios: the potential for Al mobilization expands in the Pacific Northwest of North America and high-latitude Scandinavia and declines in the Southeastern USA (figure S4). These patterns reflect shifts in S deposition over regions where soils are currently acidic.
Figure 4. Evaluated vulnerability of different soils to the present-day deposition (top panel) and increase in vulnerability under the geoengineering scenario (bottom panels, calculated as the difference in vulnerability between SSI in 2080–2100 minus present day, see also figure S4). Vulnerability is here defined as the potential of soils to release Al in the process of neutralizing incoming acidity (as shown, for instance, in [26, 27], Al is a toxic chemical for many terrestrial and aquatic ecosystems), and is calculated as the product of the aluminum saturation percentage inferred from present-day soil pH (figure S2) and the S deposition fluxes shown in figure 2, divided by its global maximum so that the index ranged between zero and 1.
Download figure:
Standard image High-resolution imageThese patterns may change over the long term given that soil pH has the potential to decline in response to acid deposition. The potential for shifts in soil pH is thought to be highest in intermediate pH soils because soils at the alkaline and acid extremes are buffered by the solubility of secondary minerals [29]. This hypothesis is supported by acid-addition experiments, which show that soil pH is most responsive to acid addition at initial pH values near 7 [30]. Since SSI also has the potential to affects soil pH, then soils that are not currently vulnerable to Al release may eventually become vulnerable. Interactions between acid deposition and alkalinity in dust might on the other hand offset the effects of deposition [27]. While the long term historical effects of dust deposition on soils are implicit in our estimates via their effect on measured soil pH, future interactions with dust may vary depending on climate and land use in dust source areas.
5. Conclusions
The danger of an unmitigated global warming is one of the most pressing issue of this century: even just considering the differences between a global warming of 1.5 °C or 2 °C show a wide range of increased risks and impact that might affect both humans and ecosystems [31]. Both of those thresholds would require a massive effort from the entire industrialized and developing world, and higher pathways of emissions (or higher climate sensitivities) that would result in higher surface warming cannot be considered unlikely.
The RCP8.5 scenario [32] is the most extreme of the IPCC emissions pathways, and this model projects it to produce global mean warming of 5 °C by the end of the century [10]. While a large sulfate injection into the stratosphere has the potential to completely offset the change in global mean temperatures from 2020, some warming would however persist in the ocean [33], and at very high latitudes [34]. Furthermore, while SSI might potentially partially alter atmospheric CO2 concentrations due to carbon cycle feedbacks [35–37], it would do nothing to reduce the amount of other GHGs [38] in the atmosphere: ocean acidification would continue to rise, and there would likely be regional shifts in the hydrological cycle [39]. If not accompanied by a strong decrease in anthropogenic emissions, SSI would have to be maintained for a long time. We can speculate that, if SSI is ever deployed, it is undertaken as a means of meeting a climate target such as the one proposed by the Paris Accord (2 °C) with a smaller amount of SO2 (as shown in figure 1) than the one considered in this study, reducing most of the climatic effects [14].
We have shown here that even such an extreme deployment of SSI, the amount of sulfate needed globally in this century would more or less compensate the reduction in anthropogenically emitted SO2 from other sources. However, the geographical distribution, and the people and ecosystems affected by it, would change from lower to higher latitudes and from largely land-focused to deposition on land and ocean. This indicates that, in order to properly assess in a holistic way the possible drawbacks of deploying SSI, more focus needs to be put not only on changes to the climate, but also on ecosystem changes. Of all possible side effects, the eventual deposition of sulfate is the most intuitive one, and in the authors' experience, one of the first concerns when members of the general public are first confronted with the idea of SSI, especially for people who might have lived through the issue of acid rain at the end of last century.
Here we have studied how different soils could be affected by the increase in acidity, with some areas in North America, Northern Europe and Oceania showing an increased potential for aluminum mobilization under geoengineering compared to present day levels, with possible consequences for local ecosystems and water reservoirs. This is just an example of possible interactions between what is injected in the stratosphere and the surface that still need to be analyzed, such as, for instance, the interaction of the new deposition with the nitrogen cycle [40], or other possible effects of the sustained accumulation of S deposition on ecosystems. This means that determining whether or not SSI could be a potential additional element of a climate change strategy requires the collaboration of scientists from a broad range of subjects, least some crucial aspects are ignored in the evaluation process.
Acknowledgments
Support for D V and D G M was provided by the Atkinson Center for a Sustainable Future at Cornell University and by the National Science Foundation through agreement CBET-1818759.