Unicellular marine microalgae (Figure 12) are grown as food for the various stages in the hatchery culture of commercially valuable shellfish. Until recently living algae constituted the sole food source for bivalve larvae and juveniles. This is now beginning to change as the result of recent research into the development of suitable non-living and artificial diets. However, the production of live algae will remain a critically important aspect of successful hatchery management into the foreseeable future, if only as a live food supplement to innovative foodstuffs.

Figure 12: Photomicrographs of two algal species commonly cultured in hatcheries, Isochrysis sp. (A) and Tetraselmis sp. (B) showing the relative difference in cell size.
Flagellate and diatom species, among the microalgae, are primary producers at the base of the marine food chain. They manufacture organic cellular components from the uptake of carbon dioxide and nutrients contained in seawater using light as the energy source in a process called photosynthesis. They are normally cultured in hatcheries in suitably treated natural seawater enriched with additional nutrients, which include nitrates, phosphates, essential trace elements, vitamins and carbon dioxide as the carbon source. Synthetic seawater may be used but it is prohibitively expensive except at the small laboratory scale.
The need to culture microalgae arises because the natural phytoplankton content of seawater used in the hatchery is insufficient to support optimum growth of high densities of larvae and juveniles reared. Particularly in the culture of larvae, the water treatments used will remove almost all of the natural phytoplankton which then needs to be replaced from cultures of preferred, high food value species. In this context, and in the provision of suitable food rations for breeding stock and juveniles, few of the very many naturally occurring algae are of good food value to bivalves and not all of these are amenable to artificial culture on a sufficiently large scale. A list of the more commonly used species in bivalve hatcheries is given in Table 1. Parameters of cell size and composition are also shown.
Table 1: The cell volume, organic weight and gross lipid content of some of the more commonly cultured algal species used as foods for bivalve larvae and spat. Species marked * are of relatively poor nutritional value.
|
Species: |
Median cell volume (µm3) |
Organic Wt. (µg 10-6 cells) |
Lipid % |
|
Flagellates: |
|||
|
Tetraselmis suecica |
300 |
200 |
6 |
|
Dunaliella tertiolecta* |
170 |
85 |
21 |
|
Isochrysis galbana |
40-50 |
19-24 |
20-24 |
|
Diatoms: |
|||
|
Chaetoceros calcitrans |
35 |
7 |
17 |
|
Chaetoceros gracilis |
80 |
30 |
19 |
|
Thalassiosira pseudonana |
45 |
22 |
24 |
|
Skeletonema costatum |
85 |
29 |
13 |
|
Phaeodactylum tricornutum* |
40 |
23 |
12 |
The culture of algae accounts for about 40% of the costs of rearing bivalve seed to a shell length of about 5 mm in a hatchery. For example, 1 million juvenile Manila clams or Pacific oysters of 5 mm shell length will consume 1 400 l of high density, cultured algae each day at the optimum rearing temperature of 24°C. Smaller daily volumes are required to feed broodstock and larvae.
The basic methods of algal culture have changed little over the years and the various steps in the process leading to production-scale cultures are introduced in Figure 13. Hatcheries have either opted for indoor, intensive culture with artificial illumination, usually external to the culture vessels, or outdoor, extensive culture in large tanks or ponds utilizing natural light. The intensive techniques are satisfactory in terms of reliability and productivity but are expensive in terms of capital outlay and labour, while the extensive methods tend to be less reliable and, sometimes not very productive. Both methods will be considered together with the essential infrastructure and methodologies. A schematic diagram of the process of culturing algae is given in Figure 14 and a floor plan of a hatchery showing the area allocated to algal culture was given earlier in Figure 5 (section 1.2).

Figure 13: Steps in the production of algae. Stock cultures (250 ml or less) remain in isolation under light and climate control (low temperature) and are only used to inoculate starter cultures when necessary. They are not aerated nor is carbon dioxide added. Starter cultures (250 ml to 4 l in volume) are grown quickly for 7 to 14 days at higher temperatures and light intensity with a supply of carbon dioxide enriched air. When ready, a small portion of the volume is used to start a new starter culture and the main portion to begin an intermediate-scale culture. Intermediatescale cultures (usually of between 4 l and 20 l in volume) may be used as food for larvae or to start a large-scale culture. Large-scale cultures are generally of a minimum of 50 l and are frequently much greater in volume.

Figure 14: The process of algal culture showing the various required inputs. Whether secondary seawater treatment is necessary or not depends on the extent to which the water is initially filtered.
Stock cultures, otherwise known as master cultures, of the preferred species are the basic foundation of culture. They are normally supplied as monospecific (uni-algal) cultures from reputable culture collections maintained by national institutions or research laboratories. Since they are valuable, they are normally kept in specialized maintenance media, for example, Erdschreiber, or alternatively in F/2 media, or on nutrient enriched agar plates or slopes, under closely controlled conditions of temperature and illumination. A special area or room off the algal culture room is usually allocated to this purpose.
Stock cultures are used only to provide lines of starter cultures (also known as inocula) when required. Every effort should be made to minimize the risk of contaminating the stock and starter cultures with competing microorganisms. The sterile procedures described below should be followed to ensure that contamination does not occur.
Stock cultures are kept in small, transparent, autoclavable containers. For example, 500 ml borosilicate glass, flat-bottomed boiling or conical flasks fitted with a cotton wool plug at the neck, suitable for containing 250 ml of sterile, autoclaved medium, are ideal. The composition and preparation of Erdschreiber medium is given in Table 2. Alternate media suitable for the purpose are Guillard’s F/2 (see Table 3) and HESAW (see Table 4). Proprietary algal culture enrichment products for addition to suitably treated seawater can also be used according to the manufacturer’s instructions. Stock cultures are also often maintained in seawater agar medium impregnated with suitable nutrients in Petri dishes or on slopes in test tubes.

Figure 15: Illuminated, temperature controlled incubators for the maintenance of small algal cultures.
Stock cultures are best kept in a cooled incubator at 4 to 12°C (according to preference), illuminated by two or more 8-watt (W) fluorescent lamps that provide a light intensity of 450 lux measured at the culture surface (Figure 15). Alternatively they can be kept in cool conditions close to a north-facing window (out of direct sunlight), or in a cool room illuminated by fluorescent lamps. The objective is not to allow rapid growth, but to maintain the cultures in good condition. The cultures are not aerated, nor is carbon dioxide introduced.
It is necessary to sub-culture stock cultures at monthly intervals to maintain them in a vigorous and healthy state. Following removal of the cotton wool plug from a stock culture flask and flaming the neck of the flask with a Bunsen burner (or butane torch), an inoculum of 20 to 50 ml is decanted into another sterile flask containing autoclaved medium. The plug is inserted after flaming of the neck of this new flask. Species name and the date are indelibly marked on the flask, which is then returned to the incubator. The original stock culture can be kept for a few weeks in the event that the new stock culture fails to grow. The stock culture transfer procedure is best performed in a cabinet that has been sterilized by ultra-violet light to further reduce the risk of contamination (see Figure 16). Details of the transfer procedure are given in the accompanying box.

Figure 16: A - schematic diagram of a culture transfer chamber. B - an autoclave suitable for the sterilization of small volumes of culture medium.
Table 2: The composition and preparation of Erdschreiber culture maintenance medium.
Constituents:
1. Seawater: Autoclave 2 l in a 3 l borosilicate glass flat-bottomed boiling flask with cotton wool plug at 1.06 kg cm-2 for 20 minutes. Stand for 2 days.
2. Soil extract: prepared as follows:
a) mix 1 kg soil from a woodland or pasture area untreated with artificial fertilizers, insecticides, etc. with 1 l of distilled freshwater;
b) autoclave at 1.06 kg cm-2 for 60 minutes;
c) decant off the supernatant liquid;
d) filter supernatant through Whatman No. 1 paper and then through a glass-fibre (GF/C) paper;
e) autoclave in 1 l aliquots in polypropylene bottles at 1.06 kg cm-2 for 20 minutes;
f) store in deep freeze until required;
g) autoclave 100 ml in 500 ml borosilicate glass, flat-bottomed boiling flask with cotton wool plug at 1.06 kg cm-2 for 20 minutes.
3. Nitrate/phosphate stock solution: Dissolve 40g NaNO3 and 4 g Na2HPO4 in 200 ml distilled water. Autoclave in 500 ml flask at 1.06 kg cm-2 for 20 minutes.
4. Silicate stock solution: Dissolve 8 g Na2SiO3.5H2O in 200 ml distilled water. Autoclave in 500 ml flask at 1.06 kg cm-2 for 20 minutes.
Procedure:
Add 100 ml soil extract (2) to 2 l of sterilized seawater (1). With sterile pipette add 2 ml nitrate/ phosphate stock solution (3) and 2 ml silicate stock (4). Decant 250 ml into 8 empty autoclaved 500 ml flasks with cotton wool plugs. Use a Bunsen burner or butane torch to flame the necks of the flasks immediately before and after decanting/pipetting. The maintenance medium is now ready to use.
|
Procedure for transferring algal cultures from flask to flask (a) Wipe all inner surfaces of inoculating booth with 85% ethanol. (b) Place all flasks that will be required in the booth; i.e. all flasks to be transferred from (the transfer flask) and flasks containing sterilized media to be transferred into (new flasks). (c) Close booth and switch on ultra-violet lamp. Leave for at least 20 minutes. (It is not safe to look directly at ultraviolet light, so a dark cover should be placed over the plexi-glass (transparent acrylic plastic) viewing plate when the light is on.) (d) Switch off lamp. Ignite small burner. (e) Remove foil caps from one transfer and one new flask. Flame the neck of each flask by slowly rotating the neck through the flame. (f) Tilt the neck of the transfer flask toward the new flask. In one motion, remove both stoppers and pour an inoculum into the new flask. Transfer approximately 50 ml for diatom species and 100 ml for flagellates. Avoid touching the necks of the two flasks. Never touch the portion of the stopper that is inserted into the flask. Once the inoculum is added, replace the stopper in the transfer flask. Slowly flame the neck of the new flask before replacing its stopper. (g) Replace foil cap over the neck of the new flask. Using a waterproof marker pen, label the new flask with the algal species inoculated and the date of transfer. (h) Repeat procedure for all flasks within the booth. Once completed, turn off burner and open booth. (i) Remove all new flasks and place in the algal incubator or a well-lit area in the algae culture facility. (j) The remaining inoculum in the transfer flasks can be used to inoculate larger cultures such as 4 l flasks or carboys. (from: Bourne, Hodgson and Whyte, 1989) |
Table 3: Guillard’s F/2 media used for culturing algae in bivalve hatcheries from Guillard (1975).
|
1. |
Nitrate |
NaNO3 |
75.0 g per l |
||
|
2. |
Phosphate |
NaH2PO4.H2O |
5.0 g per l |
||
|
3. |
Silicate |
Na2SiO3.9H2O |
30.0 g per l |
||
|
4. |
Trace Metals |
|
|
||
|
|
|
FeCl3.6H2O |
3.5 g |
||
|
|
|
Na2EDTA |
4.36 g |
||
|
|
|
|
|
||
|
Dissolve in 900 ml distilled H2O |
|||||
|
|
|||||
|
Add 1 ml of each of the following trace metal solutions |
|||||
|
|
CuSO4.5H2O |
0.98 g per 100 ml |
|||
|
|
ZnSO4.7H2O |
2.20 g per 100 ml |
|||
|
|
CoCl2.6H2O |
1.00 g per 100 ml |
|||
|
|
MnCl2.4H2O |
18.00 g per 100 ml |
|||
|
|
Na2MoO4.2H2O |
0.63 g per 100 ml |
|||
|
|
|||||
|
Make up the volume to 1 l with distilled H2O (pH ca. 2.0) |
|||||
|
|
|||||
|
Add 1 ml per litre FSW of the above solutions (#1-4). |
|||||
|
|
|||||
|
5. Vitamins |
|||||
|
|
Biotin |
1.0 mg |
|||
|
|
B-12 |
1.0 mg |
|||
|
|
Thiamine HCl |
20.0 mg |
|||
|
Dissolve in 1 l distilled H2O. Store frozen. |
|||||
|
Add 1/2 ml of vitamin solution for every 1 l of FSW. |
|||||
Table 4: HESAW media used for culturing algae in bivalve hatcheries. From Harrison et al. (1980).
|
1. |
NaNO3 |
466.7 g |
|
|
Na2.glycero.P04.5H2O |
66.7 g |
|
Dissolve in 2 1itres distilled H2O |
||
|
2. |
Na2EDTA.2H2O |
55.3 g |
|
|
H3BO3 |
38.0 g |
|
Dissolve in 1 litre hot distilled H2O |
||
|
|
||
|
3. |
FeCl3.6H2O |
1.6 g |
|
Dissolve in 100 ml distilled H20. Add 50 ml to solution #1 and the remainder to solution #2. Mix together solutions #1 and #2. |
||
|
4. |
MnSO4.H2O |
4.1 g, or |
|
|
MnSO4.4H2O |
5.4 g |
|
Dissolve in 50 ml distilled H20. Add to above solution. |
||
|
5. |
Na2MoO4.2H2O |
1.26 g |
|
Dissolve in 50 ml distilled H20. Add to above solution. |
||
|
6. |
ZnS04.7H2O |
7.3 g |
|
|
CuS04.7H2O |
1.6 g |
|
Dissolve in 100 ml distilled H20. Add 10 ml of solution to above solution. |
||
|
7. |
Na2SeO3 |
0.173 g |
|
Dissolve in 1 l distilled H20. Add 1 ml of solution to 100 ml distilled H20 to make stock solution. Add 10 ml stock solution to above solution. |
||
|
Bring volume of solution to 10 l by adding distilled H20. Autoclave before use. Add 1 ml of solution for every 1 l of FSW. |
||
|
8. |
Na2SiO3.5H2O |
224.0 g, or |
|
|
Na2Si03.9H2O |
300.0 g |
|
Dissolve in 1 l distilled H20. Slowly add 1.5 l of 1 Molar HCl (133.5 ml concentrated HCl in 1.5 L distilled H20). Bring volume of solution to 10 l by adding distilled H20. Autoclave prior before use. Add 1 ml of solution for every 1 l of FSW. |
||
|
9. |
Vitamins |
|
|
(Follow directions for vitamins in Table 4.) |
||

Figure 17: Photographs showing typical facilities for maintenance of starter cultures.
Procedures for the maintenance of starter cultures (inocula) are almost identical to those described above. These cultures are specifically grown to provide inocula to start larger volume cultures needed to produce food.
A line of starter cultures is originally set-up from the stock culture of the required species. Starter cultures, like the stocks, can be grown in 500 ml boiling flasks in 250 ml of culture medium. Because they are needed to provide inocula it is necessary to grow them quickly. They are grown at 18 to 22°C at a distance of 15-20 cm from 65 or 80 W fluorescent lamps, giving a level of illumination at the culture surface of 4 750 to 5 250 1ux (Figure 17). Starter cultures are generally aerated with an air/carbon dioxide (CO2) mixture.
Starter cultures are grown for variable periods of time prior to use. In the case of diatom species, which have short generation times, this period is from 3 to 5 days. For the majority of flagellates it is 7 to 14 days. When ready for use a starter culture is sub-cultured using sterile techniques, as previously described. Twenty to 50 ml, (depending on species and the density of the culture), is transferred to a fresh 250 ml culture - to maintain the starter culture line. The remainder is used as an inoculum for larger cultures (up to 25 l in volume) to be grown for feeding or as an intermediate step in the process of large-scale culture, where they in turn act as the inocula for much larger cultures.
Larger volume starter cultures may be needed to inoculate large-volume production cultures. For clarity, cultures of between 2 and 25 l volume will be referred to as intermediate-scale cultures. As an example, a 200 l production culture will initially begin with a 250 ml starter of the required species which is then transferred when it has grown to a larger volume 2 to 4 l starter. When a 200 l culture is about to be started, 200 to 400 ml of the 2 to 4 l starter culture is used to start a new 2 or 4 l starter culture and the remainder to start the 200 l production culture.
With larger volume starters it is advantageous to increase the level of illumination and to aerate the culture with an air/carbon dioxide mixture. It is advisable to dilute the medium to grow diatom species to a salinity of 20 to 25 PSU (practical salinity units, equivalent to parts per thousand) to obtain the best possible growth rates. Most flagellate species are best grown at about 30 PSU.
Most laboratories and hatcheries requiring small volumes of algae for food use spherical glass flasks or glass or clear plastic carboys of up to 25 l volume (Figure 18). These are generally operated as batch culture systems or semi-continuously. Batch culture involves the inoculation of the culture medium with the required species. The culture is then grown rapidly until a further increase in cell density is inhibited by the failure of the light to adequately penetrate the culture, The culture is then completely harvested, the container washed and sterilized and started again with a new culture.

Figure 18: Two different approaches to the intermediate-scale culture of algae: A - 20 l volume round flasks; B - using equally as effective wine making carboys of 15 to 20 l volume.
The semi-continuous method involves starting the cultures in the same way but instead of completely harvesting them when they have grown, they are partially harvested before the light limiting stage is reached. The harvested volume is then replaced with freshly prepared culture medium and the process repeated 2 or 3 days later. In this way the life of a culture is extended. With some of the hardier species, e.g. Tetraselmis suecica, cultures will last for 3 months or more with harvests of 25 to 50% of the culture volume 3 times each week. Batch culture is generally used for delicate species and the rapidly growing diatoms. Semi-continuous culture is mainly used with hardier species of flagellates.
Harvesting takes place in semi-continuous culture during the exponential phase of growth. Batch harvests are made generally at the peak of exponential growth as the cultures enter the stationary phase. An illustration of the meaning of these terms is given in Figure 19. In this case the species cultured is the large, green flagellate, Tetraselmis.

Figure 19: Phases in the growth of algal cultures illustrated by a typical growth curve for the large, green flagellate, Tetraselmis suecica.
At inoculation from the starter culture, the starting cell density in the culture is 25 to 50 cells per ml (cells per microlitre). After inoculation these cells grow and divide increasingly rapidly as they acclimatize to the culture conditions. This acclimatization period, which lasts for 2 to 3 days, is called the lag phase. Once adapted to the conditions, the rate of cell division accelerates and increase in the number of cells in the culture is logarithmic. This period lasts for 4 to 6 days and is called the exponential growth phase. Cell division rate then slows as light penetration through the culture and/or nutrients become limiting. The culture then enters the stationary phase, which can last for many days in the case of flagellates or only for a short time for diatoms. Cultures of flagellates remain in this phase by the recycling of nutrients from dead and decaying cells, but in the case of diatoms, which may produce self-inhibiting metabolites, which attract bacterial growth, the culture collapses.
In the example shown in Figure 19, batch cultures of Tetraselmis would be harvested at a density of about 2 000 cells per.l and semi-continuous cultures at about 1 500 cells per.l. These densities can be increased, within limits, by increasing the light intensity falling on the cultures, by maintaining the pH at between 7.5 to 8.2 with controlled CO2 input and by the addition of extra nutrients as the culture density increases.
The complexity of the culture operation depends on the requirement for algae and the cost constraints within which the system needs to operate. In the simplest form the culture system may be just a scaled-up version of the starter cultures, using 2 l to 25 l flat-bottomed, glass flasks or carboys. These are part filled with the culture medium - in this case sterile, nutrient-enriched seawater - and then they are inoculated with the required species and aerated with a mixture of 2% CO2 carried in compressed air. The carbon dioxide is from a bottled gas source with gas pressure and flow regulation. This is to provide the carbon source for photosynthesis and to control pH within the range 7.5 to 8.2. The air/CO2 mixture is filtered through a 0.2.m porosity cartridge or membrane filter to remove the majority of air-borne contaminants and competing microorganisms. Examples of this type of system are illustrated in Figure 18. The culture medium is prepared from filtered or sterilized seawater.
There are various options for culture water treatment:
a) either the seawater is filtered to remove bacteria using 0.22 or 0.45.m membrane cartridge filters, or,
b) it is batch or continuously pasteurized at 65 to 75°C or,
c) it is autoclaved at 1.06 kg per cm2 for 20 minutes (After autoclaving the medium must be allowed to stand for 2 days in a suitable container closed from the atmosphere). Or,
d) it is chemically sterilized with sodium hypochlorite solution at 25 mg per l freechlorine (by adding 0.5 ml of domestic bleach - 5% sodium hypochlorite - per l of filtered seawater). Before use, the residual free-chlorine is neutralized by adding an excess of sodium thiosulphate solution (50.0 mg per l) prepared in distilled water.
Note: Methods (a) and (c) are most commonly used for small-scale culture preparation; (b) and (d), after prior filtration to 1 or 2 µm particle size, for large-scale culture.
After the sterilizing treatment, nutrient additions are made. Details of the nutrient enrichment used at the Ministry of Agriculture, Fisheries and Food, Fisheries Laboratory, Conwy, UK, which is suitable for all of the commonly cultured species, is given in Table 5. Note that diatoms require the addition of silica (Si) to the basic nutrients. The medium is then ready to dispense aseptically to the culture flasks, which are then ready to be inoculated. In recent years, several proprietary brands of algal culture nutrients have become available. These are generally based on the Guillard F/2 formula and provide excellent growth results (see Tables 3 and 4 for the basic formulae).
To obtain the maximum productivity of most species it may be necessary to dilute the seawater with pure (distilled) freshwater (or from an uncontaminated source) before filtration or autoclaving. Growth and cell division rates of Chaetoceros calcitrans, Thalassiosira pseudonana and Skeletonema costatum are optimal at a salinity of about 20 to 25 PSU. Productivity of many of the flagellates is optimal at 25 to 30 PSU.
Illumination for culture growth is provided by fluorescent lamps, usually mounted externally to the culture flasks (see Figure 18). The number of lamps used is determined by the height and diameter of the culture vessels with the object of providing 15 000 to 25 000 lux measured at the centre of the empty culture container. Two 65 or 80 W lamps are sufficient to illuminate 3 l glass flasks, which are about 18 cm diameter, whereas 5 lamps of the same light output are necessary for vessels of about 25 l volume (35 cm diameter). Growth is optimal at 18 to 22°C for most species.
Table 5: Nutrient salt stock solutions for the enrichment of diatom cultures in treated seawater. The addition of stock solution C is omitted in the culture of flagellates.
|
Stock A |
||
|
FeCI3. 6H20 |
|
1.30 g* |
|
MnCl2.4H20 |
|
0.36 g |
|
H3BO3 |
|
33.60 g |
|
EDTA |
|
45.00 g |
|
NaH2PO4.2H20 |
|
20.00 g |
|
NaNO3 |
|
100.00 g |
|
Trace metal solution* |
|
1.0 ml |
|
Distilled water |
to |
1000 ml |
|
Add 2 ml Stock A per litre of filtered seawater |
||
|
* Trace metal solution |
||
|
ZnCI2 |
|
2.10 g |
|
CoCI2.6H2O |
|
2.00 g |
|
(NH4)6Mo7O24.4H2O |
|
0.90 g |
|
CuS04.6H20 |
|
2.00 g |
|
Distilled water |
to |
100 ml |
|
Acidify with sufficient conc. HCI to obtain a clear solution. |
||
|
*Amount for enrichment of autoclaved seawater. For filtered seawater use 3.25 g. |
||
|
Stock B |
||
|
Vitamin B12 (Cyanocobalamin) |
|
10 mg |
|
Vitamin B1 (Aneurine hydrochloride) |
|
200 mg |
|
Distilled water |
to |
200 ml |
|
Add 0.2 ml Stock B per l of filtered seawater |
|
|
|
Stock C |
|
|
|
Na2SiO3.5H20 Distilled water |
to |
4.0 g 100 ml |
|
Add 2 ml Stock C per l of filtered seawater. |
||
Examples of cell density achieved in the small-scale culture of a number of nutritionally important species are given in Table 6. These are values obtained at the MAFF Fisheries Laboratory, Conwy, and are typical of densities achieved elsewhere in commercial culture enterprises. It is interesting to note that much higher cell densities of Chaetoceros calcitrans are obtained in 2 l than in 20 l cultures. This does not necessarily mean that productivity in terms of biomass is lower. In all cultured species the size of cells is variable according to culture conditions and the growth phase. In 2 l cultures of Chaetoceros higher cell densities are reached but the individual cells are smaller: 35 µm3 compared with 50 µm3 in 20 l cultures. The dry weight content is also lower at about 10 µg per million cells (micrograms per million cells) compared with up to 18 µg per million cells in 20 l cultures. Other species show similar variability in size related parameters depending on cell density and conditions, quite apart from the inherent differences in cell size between species.
Through manipulation of culture conditions of the larger species, such as Tetraselmis, it is feasible to alter cell size so that the cells can be more readily ingested by smaller larvae. Small-scale culture systems can be technically improved to increase their performance by operating them as chemostats. But, if the objective is solely to produce more food, the better solution is to turn to large-scale culture methods.
Table 6: Cell densities at harvest (cells µl-1) achieved in small-scale batch (B) and semi-continuous (SC) 2 l or 20 l cultures for a selection of nutritionally valuable species. The salinity of the culture medium is also given.
| |
Culture Conditions |
Harvest density (cells µl-1) |
||
|
Species |
Volume (l) |
Type |
Salinity (PSU) |
|
|
Isochrysis (T-ISO) |
20 |
SC |
25 |
15 000 |
|
Tetraselmis suecica |
20 |
SC |
30 |
2 000 |
|
Chaetoceros calcitrans |
2 |
B |
20 |
60 000 |
| |
20 |
B |
20 |
22 000 |
|
Thalassiosira pseudonana (3H) |
2 |
B |
20 |
40 000 |
Before discussion of large-scale culture methods, a short description relating to the estimation of the density of cells in cultures at any scale is warranted. Various methods are available to estimate algal cell density including the use of spectrophotometers, fluorometers, haemocytometers, and Coulter counters ("multisizers").
A spectrophotometer or fluorometer measures the chlorophyll A content in an algal culture and this can be used to obtain a quick approximation of cell density. Graphs comparing cell density and readings on either instrument must be prepared for each algal species. However, the chlorophyll A content in an algal cell is not constant and varies with nutritional state of the cell. This will affect the accuracy of cell density estimates derived when using these instruments.
More accurate estimates of cell density can be made using a haemocytometer or a Coulter Counter.
Haemocytometers are thick glass slides with two chambers on the upper surface, each measuring 1.0 x 1.0 mm. A special coverslip is placed over these two chambers giving a depth of 0.1 mm making the total volume of each chamber 0.1 mm3. The base of each chamber is marked with a grid to aid in counting cells within the area (Figure 20). Prior to counting motile algal species, 1 or 2 drops of 10% formalin should be added to a 10 to 20 ml sample of the culture to be counted. With the coverslip in position, one or two drops of the algal sample are introduced by means of a Pasteur pipette to fill both chambers.

Figure 20: Diagram of the grid marked on a haemocytometer slide.
Cell density is estimated as follows. The central grid of each chamber (outlined in blue in Figure 20) is sub-divided into 25 squares (also outlined in blue in the diagram), each measuring 0.2 x 0.2 mm. Each larger square is further sub-divided into 16 smaller squares measuring 0.05 x 0.05 mm. The numbers of cells in 10 randomly chosen 0.2 x 0.2 mm squares are counted and the average or mean is calculated. This gives the mean number of algal cells per 0.2mm x 0.2mm x 0.1mm, or 0.004 mm3.
|
Example: A. Counts of algal cells: 40 + 30 + 50 + 60 + 55 + 65 + 70 + 45 + 40 + 70 = 525 Average = 52.5 cells per 0.004 mm3 B. Multiply the average by 250 to give the average number of cells per mm3. C. Since there are 1000 mm3 in 1 ml, multiply the value calculated in B by 1 000. In this example, the cell density would be 52.5 x 250 x 1 000 = 13.1 million (13.1 x 106) cells per ml. Note: 1 cell per ml (cells ml-1) = 1 000 cells per µl (cells µl-1) |
An easier and more accurate method of estimating cell density is to use a Coulter Counter (now called a "multisizer" - see Figure 21). This instrument was developed primarily for counting blood cells.
Several models are available and all operate on the same principle. A small electrical current is passed between two electrodes. Each time a cell passes between them, the current is impeded and the cell is counted. The size of the aperture tube is important, and for counting algal cells of between 2 to 10 µm an aperture of 50 or 100 µm in diameter is required. A known volume of water is drawn through the opening in the aperture tube and the cells are counted. More detailed explanations of the operation of a Coulter Counter are available and are included in the suggested reading material at the end of this section.
Since algal cultures are often dense, samples must be diluted to a density that can be counted accurately when using an electronic counter - approximately 50 000 cells per ml (50 cells per µl). Algal samples are usually diluted with a 3% solution of sodium chloride (by dissolving table salt in distilled water) or with 0.45 µm membrane filtered seawater.
|
Example: Add 0.2 ml of algal culture to 20 ml 3% NaCl. Mix well. Count 3 times and obtain a mean value. Individual counts = 5 280; 5 336; 5 120. If the volume of the solution sampled by the Coulter counter is 0.1ml, then the average = 5 245 cells per 0.1 ml. Multiply 5 245 by 10 to obtain the number of cells in 1 ml of the sample, and multiply by 100 to correct for the dilution factor. In this example, cell density would be 5 245 x 10 x 100 = 5.2 million (5.2 x 106) cells per ml. |

Figure 21: Electronic particle counters used in hatcheries for counting the density of cells in algal cultures. A - a Coulter Counter; B - a Beckman Multisizer; C - details of the sample chamber of a Coulter Counter showing the aperture tube inserted in a sample container.
Electronic counters and particle sizers are expensive but used machines can be purchased for a reasonable price. The cost of purchase is quickly offset by the time saved and the accuracy of the counts.
Commercial bivalve hatcheries need to produce large volumes of good quality, high-food-value algae daily to support economic-scale seed production. Examples of some of the systems currently in use in Europe and North America are described in this section. They range from simple polyethylene bags either suspended, or supported by a cylinder of plastic coated or galvanized steel mesh, to sophisticated electronically operated turbidostats. All have the common feature that the culture is contained in a tall, narrow cylinder, this being the most efficient configuration. Culture in rectangular (Figure 22) or circular tanks with overhead illumination is largely obsolete. The exception is in hatcheries, mainly on the west coast of North America. They continue to use large, circular tanks illuminated by high output, metal halide lamps. Highest productivity is achieved by mounting the illuminating lamps internally in the cultures (Figure 23) rather than externally as a bank of fluorescent lamps.

Figure 22: Large-scale culture was often in large, circular or rectangular tanks with overhead illumination. This format has been largely superceded by the use of tall, cylindrical vessels.

Figure 23: Efficient 200 l volume, water cooled, internally illuminated algal culture tanks. A - harvesting a culture by siphon. B - details of construction. The outer fibreglass jacket has cooling tubes moulded to the outer surface to help dissipate the heat from the internally mounted fluorescent lamps. C - details of the lid of the vessels with its stoppered media inlet; cotton wool packed air exhaust at the back; an access/observation port and showing the top of the inner acrylic cylinder. The cables of the 6, 150 cm length fluorescent lamps are routed through a central, PVC core tube which also supports the clamps that hold the lamps in place.
Polyethylene can be purchased as heavy gauge, "lay-flat" tubing in various widths and in rolls containing convenient lengths. By cutting a suitable length and heat-sealing one end a sterile, flexible culture container can be formed, either as a cylinder or as an oblong bag. Containers formed in this way can be strengthened by supporting them within a plastic or plastic coated steel mesh frame. Alternatively, the cylinders can be suspended, with or without lateral support mesh, if the diameter of the bag is less than 30 cm and the height less than 200 cm. Examples are shown in Figure 24.

Figure 24: Examples of polyethylene bag and solar grade, fibreglass cylinder algal culture systems: A - 480 l polyethylene bags supported inside steel mesh frames and illuminated by natural light within a greenhouse. B - 80 l bags suspended around a circular central framework on a rotating, ceiling mount. Fluorescent lamps are attached to the central framework. C - plastic mesh supported, oblong polyethylene bags mounted either side of banks of fluorescent lamps. D - 100 l solar grade, fibreglass cylinders against a bank of vertically mounted fluorescent lamps. E - 2.4 m high, 0.3 m diameter fibreglass cylinders, externally illuminated by vertically mounted 2.4 m length fluorescent lamps.
Bags are the least expensive way of constructing large-scale culture vessels and such containers can be used indoors with artificial illumination or outdoors taking advantage of natural light. The bags illustrated in Figure 24A are formed from lengths of 10 000 gauge, extra heavy duty, 90 cm width polyethylene tubing. They are supported by welded steel mesh frames and have a capacity of 480 l with a large surface area of 3.2 m2 for light penetration. Large cultures of this type can be illuminated by vertically mounted 1.8 m long, 80 W fluorescent lamps or can be sited outdoors, out of direct sunlight. Bag systems shown in Figure 24B and C are formed from the same material but are supported by sturdy, plastic mesh.
In general, the larger the diameter of the culture vessel, the lower is the maximum cell density possible with a fixed level of illumination. Nevertheless, these bags are superior in productivity to similar volume rectangular, fibreglass or plastic tanks sometimes still used for mass culture. They are, however, inefficient when compared with internally illuminated cultures as can be seen in yield data given in Table 7.
Polyethylene bag cultures have a relatively short life because the internal surface attracts culture debris and bacteria, which collectively reduce light penetration and are a source of contamination. At the end of a culture run it is necessary to renew the bag. Large diameter bags are inefficient but those less than 30 cm diameter can be effective because the surface area to volume relationship for light penetration is improved.
A more permanent solution to the same principle is offered by solar grade, transparent fibreglass sheet that can be formed into a cylinder and solvent welded or purchased in cylindrical form. The light penetration qualities of this material are excellent and the vessels so constructed are durable. Cylinders that are 150 to 240 cm high and 30 to 50 cm in diameter are commonly used in North American hatcheries (Figure 24D and E).
Table 7: Comparison of yields of Tetraselmis and Phaeodactylum from various large-scale culture systems. Yield is calculated as litres per day at a standard cell density per litre of culture volume. (* Internally illuminated systems). References quoted are given in full in the suggested reading list at the end of this section.
|
Species/System |
Reference |
Yield |
|
Tetraselmis |
|
|
|
80 l turbidostat* |
Laing & Jones, 1988 |
1.25^ |
|
200 l vessels* |
Laing & Helm, 1981 |
0.40 |
|
340 l tanks |
Griffith et al., 1973 |
0.12 |
|
Phaeodactylum |
|
|
|
200 l vessels* |
Helm & Laing, 1981 |
0.35 |
|
20 l flasks |
Ukeles, 1973 |
0.33 |
|
480 l polyethylene bags |
Baynes et al., 1979 |
0.15 |
|
195 l cylinders |
Wisley & Purday, 1961 |
0.06 |
* A yield value of 1.25 indicates an average daily harvest of 100 l at standard cell density from an 80 l volume culture.
Internally illuminated culture vessels are costly to construct but inexpensive to operate. By mounting the lamps inside a glass or clear plastic cylinder, as in Figure 23, the effective distance for the light to penetrate the culture is greatly reduced. In the example shown, the culture vessel is 150 cm high by 40 cm diameter. The internally mounted light cylinder is 15 cm diameter, therefore, the light energy emitted by 6, 80 W, 150 cm length fluorescent lamps travels only about 14 cm to the perimeter of the culture. In a later development, this distance was further reduced in smaller 80 l culture vessels and yet the same total productivity was achieved as in 200 l cultures.
Productivity (or yield) is determined as the total number of algal cells harvested from a culture each day. Internally lit cultures have a long life, some lasting for more than 100 days with the hardier species. When a culture is finished, the vessel is sterilized by filling with 20 to 50 mg per l (free-chlorine) hypochlorite solution, allowing it to stand for at least an hour, before thorough rinsing with culture quality, filtered seawater, after which it is drained and re-started.
Basic conditions of culture are essentially the same as previously described. The main difference is in the treatment of water to be used as the culture medium. Autoclaving or sub-micron filtration is too expensive for the large volumes necessary. Seawater filtered to 1 or 2 µm particle size by cartridge filtration is acceptable for some of the larger celled species, e.g. Tetraselmis and Skeletonema. Otherwise, pasteurization or chemical sterilization is recommended. Control of salinity and pH is required and, to obtain maximum productivity, the intensity of illumination must be carefully calculated for the diameter of the culture vessel.
The objective in culture management is to obtain the greatest possible daily yield of algae so that the culture systems are operated cost effectively. This yield must be sustained for long periods of time to maintain the hatchery output of juveniles. Ineffective management of algal culture greatly influences the potential for production and ultimately the selling price of the bivalve seed.
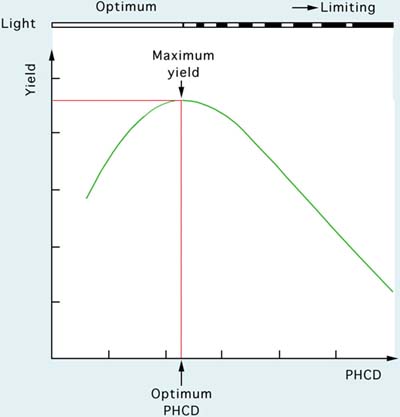
Figure 25: The relationship between the productivity of a culture system (yield) and light energy input. See text for explanation.
The operation of semi-continuous, internally illuminated cultures will be considered in this section. The general principles are applicable to any culture facility and at any scale of production. The basic yield relationship with light energy input is shown in Figure 25. Yield is calculated as the number of litres of algae harvested per day at a standard cell density per µl.
The use of the term standard cell density requires explanation. In order to compare yields of different species in a culture system, a common factor based on dry weight biomass of harvested algae is applied. Different alga species vary widely in linear dimensions and in weight per cell, as already seen in Table 1. Knowing the weight per cell, an equivalent number of cells can be calculated for each species to provide a given biomass. For some of the important species this approximates to:
250 cells of Chaetoceros calcitrans = 100 cells of lsochrysis galbana = 60 cells of Skeletonema costatum = 10 cells of Tetraselmis suecica, on a dry weight basis.
Thus, for Skeletonema and Tetraselmis the standard cell densities used in yield calculation are 6 000 and 1 000 cells per µl respectively (alternatively, 6 million and 1 million cells per ml).
Another term requiring explanation is the concept of post-harvest cell density (PHCD).
PHCD = cell density per unit volume (cells per µl) immediately after daily harvesting and replacement of the volume removed with fresh culture medium.
It is the cell density (following harvesting and replenishment of the culture volume with new medium) relative to light intensity that will largely dictate growth of the culture in the following 24-h period. Reference to Figure 25 shows that yield is at a maximum at the optimum PHCD when light energy input is not limiting. At PHCD values below the optimum, cell division rate (K), described by the equation:
|
|
|
(Nt = cells per µl at
harvest) |
is at its maximum but the PHCD is too low for maximum productivity. Above the optimal PHCD, light becomes increasingly limiting due to the self-shading effect of cells at higher culture densities. Photosynthesis decreases, therefore, cell division rate and daily yields decrease. Yield is maximal at a particular light intensity and can be increased or decreased by altering the light energy input.

Figure 26: The effect of light intensity on yield of Tetraselmis in 200 l internally illuminated culture vessels.
The effect of increasing light intensity in 200 l cultures of Tetraselmis by increasing the number of 80W fluorescent lamps from 4 to 8 is shown in Figure 26. Four lamps provide an illumination intensity of 7.6 mW per cm2 (7.6 milliwatts per centimetre squared which provides an illumination intensity of 28 000 lux) and 8 lamps, 14.0 mW per cm2 (52 000 lux). Maximum yields, increased from 67 l per day at 1 000 cells per µl at 28 000 lux to 96 l per day at the same cell density at the highest illumination intensity. Improvements in yield result from an accelerated cell division rate and, because of the greater light energy input, cultures can be operated at a higher PHCD. Yields from 8 and 6 lamp units are similar. This is because the cultures approach light saturation at the highest illumination level, therefore, yield relative to cost of the extra energy input decreases with 8 lamp units.
The influence of PHCD on cell division rate (K) in Tetraselmis in 200 l cultures is shown in Figure 27A. Increasing PHCD values result in exponentially decreasing K values, as light becomes progressively more limiting. Data in Figure 27B and C show that values of K decrease, therefore, yield decreases with increasing pH and salinity. This highlights the need to control these parameters by (a), increasing carbon dioxide input in the case of increasing pH and (b), by diluting the culture medium in the case of elevated salinity. Devices to control pH by varying the rate of input of carbon dioxide are available from suppliers of aquaculture equipment.

Figure 27: Effects of A - post-harvest cell density (PHCD) and B - pH on cell division rate, and the influence of salinity on the productivity of cultures of Tetraselmis suecica - C.
Culture techniques that improve maximum yield can also have the effect of altering the size of cells at harvest (Figure 28). With increasing PHCD and onset of light limitation, cells decrease in size measured as either dry or organic weight. However, within normal operating limits of PHCD the overall effect on maximum yield, on a biomass basis, is small.
The nutrient content of the culture medium also has an important effect on the maximum yield achievable in large-scale culture systems. This is shown in Figure 29, which provides data on the culture of the diatom, Skeletonema costatum. Diatoms require silica, which is provided in the form of SiO3-Si, to allow development of the silicaceous frustrules that enclose the cytoplasm. If silica is limiting, cell growth and division rates decrease and yields diminish. This is clearly shown in the comparison of 6, 80 W fluorescent lamp units at 30 mg per l Si (Figure 29A) and at 5 mg per l Si (Figure 29C). Cultures at 30 mg per l Si provided a maximum daily yield of 160 l (from a culture volume of 200 l at 6 000 cells per µl), whereas at 5 mg per l the maximum yield was only 74 l - less than from a 4 lamp unit at the highest Si level (Figure 29B). The maximum yield (Figure 29) is considerably greater than was obtainable from efficiently operated cultures of Tetraselmis and reflects the much higher cell division rates hence, productivity achievable with diatoms.
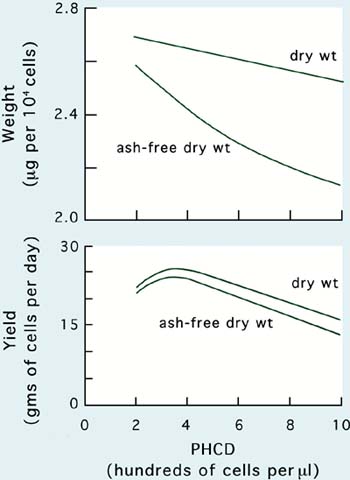
Figure 28: Relationships between post-harvest cell density (PHCD) and the size of cells in terms of weight and the productivity of semi-continuous culture of Tetraselmis suecica.
So far discussion has centered on semi-continuous culture methods. Although less labour intensive than batch harvested systems, the manpower component in operating a daily harvesting schedule is still relatively high. As a consequence it is usual to decrease the harvesting rate to 48-h intervals. This is achieved by operating the cultures at a lower PHCD. Nevertheless, the point of maximum yield can be reached within the 48-h interval so that light limitation will influence overall productivity. The solution is to operate the cultures continuously, i.e. continuous harvesting. This solution is feasible using optoelectronic control of cell density. A diagram of an automated system developed and used at the Fisheries Laboratory Conwy, UK is shown in Figure 30.

Figure 29: Relationships between post-harvest cell density and yield at standard cell density of Skeletonema costatum cultures operated semicontinuously at two light intensities and silicate concentrations.

Figure 30: Schematic of a continuous ("turbidostat") culture system (not drawn to scale). Key: 1, reservoir of seawater medium (200 l volume); 2, peristaltic pump; 3, resistance sensing relay (50 to 5 000 ohms); 4, light dependent resistor (ORP 12); 5, cartridge filter (0.45 µm); 6, culture vessel (80 l volume); 7, six, 80 W fluorescent lamps; 8, collecting tank to receive the harvest (125 l volume).
The key component in this system is a Light Dependent Resistor (LDR) clamped onto the outer surface of a transparent culture vessel. Light falling on the LDR after penetrating the culture varies depending upon the density of cells in the culture. Internal illumination is used, as in the semi-continuous, large-scale systems previously described. As cell density increases the light transmittance through the culture decreases and this increases the resistance value of the LDR. This increase is detected by a Resistance Sensing Relay (RSR) which is set to activate a peristaltic pump when a certain pre-set resistance value is reached. The RSR is adjusted to operate at the light intensity at which cell division is at a maximum. When activated, the peristaltic pump supplies fresh medium to the culture vessel and this displaces an equal volume of the culture into a receiving container. As the culture in the vessel becomes increasingly diluted, the transmittance of light through the culture, detected by the LDR increases, the resistance of the LDR decreases, and the RSR switches off the peristaltic pump.
This apparatus is inexpensive to construct with modern electronics and is very effective in maintaining cultures at peak productivity. Yields from an automatic, 80 l system for Isochrysis galbana (Clone T-Iso) and Tetraselmis are similar to those from the larger, 200 l units operating semi-continuously. A maximum yield of Tetraselmis of about 100 l per day at 1 000 cells per µl is achievable by operating the automatic system at about 2 000 cells per µl. Yields of about 90 l per day at 10 000 cells per µl have been obtained with lsochrysis operating at a culture density of 16 000 cells per µl.
The principle of automatic operation is not new. Chemostats or turbidostats using external light sources for the production of microalgal species have previously been described. The Conwy system described above is an updated and more efficient version of the concept. Continuous culture systems based on either vertically or horizontally arranged, polyethylene bag units are commercially available.
Cultures will fail to grow, will become overly contaminated with competing microorganisms or will crash even in the best-run hatcheries. Below are some pointers to check to determine the source of such failures.
1. Air supply. Is there adequate air entering the cultures? Are the cells sedimenting to the bottom of the culture vessel? This may happen when culturing certain diatoms, in which case the air flow rate should be increased. It should not happen in the case of commonly cultured flagellates. If it does, then the problem lies elsewhere.
2. Temperature. Check min/max thermometer. Were there any increases or decreases in the temperature of the algal culture facility over the past 24 hours? Most of the commonly cultured algal species cannot tolerate temperatures above 26ºC for extended periods - or temperatures below 12ºC. Temperatures in the range 18 to 22ºC are ideal.
3. pH. Check CO2 supply. Is the CO2 cylinder empty? Check pH of the algal cultures using a pH probe. Is the pH too high (above 8.5)? Is the pH too low (below 7.5)? Adjust the CO2 supply accordingly.
4. Nutrients. Check records for the last time the cultures received nutrients. This is particularly important for semi-continuous cultures.
5. Contamination. Are the walls of the culture container, particularly at the water/air interface, visibly foaming or fouled with what appears to be detritus? If so, the culture is at the end of its useful life and needs to be replaced. If this is a continuing problem in the early stages of the culture cycle with a particular species, then check the starter cultures for signs of contaminating organisms and replace them as necessary.
Not all species can be cultured successfully during the entire season. Some have particular "windows of opportunity" when they can be grown reliably. However, there is no consistency between hatcheries as to when a named species will grow well or will not. This has to be learned through on-site experience and highlights the importance of maintaining thorough records.
Intensive culture systems, described above, are closely controlled and highly productive, providing food for larvae, small juveniles and hatchery-held broodstock. An alternative, particularly suited to the provision of food for larger juveniles, is extensive outdoor tank culture, which makes use of natural light (Figure 31). This involves the fertilization of a large volume of seawater with the basic nutrients necessary for production, namely nitrogen, phosphorus and silica in one form or another. Here, the objective is not necessarily to induce a monospecific bloom, but a mixed flagellate and diatom population at a density greater than would normally occur in the sea.
It is possible to induce monospecific blooms by prior fine (<2 µm particle retention) filtration of the impounded seawater and the introduction of an inoculum of the required species, as long as it is hardy and vigorous. The use of seawater, or suitably saline brackish water, drawn from wells will also serve this purpose. However, it is difficult to maintain such blooms for long periods because they rapidly become contaminated with other microorganisms.
Multispecific blooms are more easily managed and rely on the natural phytoplankton content of the seawater utilized as the inoculum. While the species composition will vary from one blooming to another, according to season and environmental conditions, algae produced in this manner is nutritionally valuable in growing juveniles and also for maintaining broodstock.

Figure 31: Examples of large-scale, outdoor algal production. A - circular, covered, semitransparent, fibreglass tanks at a hatchery in British Columbia, Canada; B - 450 000 l concrete tanks used to bloom natural phytoplankton in support of spat culture at the Fisheries Laboratory, Conwy, UK; C - large concrete tanks with sloping bases used for monospecific algal production at Turpiolito, Venezuela: D - 2,500 l fibreglass "fish boxes" at a hatchery in Nova Scotia, Canada.
At the Fisheries Laboratory, Conwy, large, outdoor concrete tanks ranging in volume from 60 m3 to 450 m3 have been used for extensive algal production in support of the nursery culture of bivalve seed. These tanks are filled with seawater of 28 to 32 PSU salinity from the adjacent estuary at approximately 2-week intervals. In this form of culture, fertilizers are added 3 days before the tank is needed to produce algae as food for juvenile bivalves. The chemicals added are:
|
Urea NH2CONH2 |
(46% N) |
1.50 g per m3 |
|
Triple superphosphate P2O5 |
(20% P) |
1.56 g per m3 |
|
Sodium metasilicate Na2SiO3.5H20 |
(13% Si) |
10.60 g per m3 |
Concentrations of NH2N are 50 µg atoms per l; PO4-P, 10 µg atoms per l and of SiO3-Si, 50 µg atoms per l. More crudely, the application of poultry or other animal manure at 500 kg per hectare for tanks and ponds of about 1 m depth can be an effective and less costly source of nutrients.
The rate of development of a bloom is related to the initial species composition and density of algae in the seawater, day length, the amount of incidental illumination falling on the surface of the water, nutrient levels and temperature. The surface area/volume relationship of the tank or pond is important. Shallow tanks and ponds of about 1 m depth are more effective than deeper water, permitting better light penetration. Aeration of the tanks or ponds is beneficial to production.
The duration of the bloom depends on a number of factors related to the species of algae that develop in the prevailing conditions and the rate at which the algae are grazed by the bivalves. Usually, a bloom of useful density for feeding purposes can be maintained for 7-10 days after which the tank is drained, cleaned and refilled with fresh seawater.
The species composition of blooms can be manipulated to some extent by altering the types of fertilizers added. For example, by omitting Si, flagellate species may dominate because the natural Si content of the water upon which diatoms depend will rapidly become depleted. In smaller tanks it is possible to inoculate the fertilized water with a species grown in intensive culture systems. Whether or not this species will become dominant in the bloom depends on environmental conditions and the presence or absence of competing species. In general, the use of artificial fertilization of impounded seawater is a valuable technique in bivalve culture, particularly in nursery systems for juveniles. It is often possible to improve phytoplankton production by a factor of 5 or more compared with open sea conditions. The cost in fertilizers is small per 1 000 l of seawater compared with the considerable benefits in the increased commercial value of the faster growing juveniles.
Baynes, S.M., Emerson, L. & Scott, A.P. 1979. Production of algae for use in the rearing of larvae fish. Fish. Res. Tech. Rep., MAFF Direct. Fish. Res., Lowestoft, 53 (3): 13 - 18
Bourne, N., Hodgson, C.A. & Whyte, J.N.C. 1989. A Manual for Scallop Culture in British Columbia. Canadian Tech. Rep. Fish and Aquatic Sciences, No. 1694: 215 pp.
Droop, M.R., 1975. The chemostat in mariculture. In: G. Persoone and E. Jaspers (eds) Proceedings of the 10th European Symposium on Marine Biology, Ostend, Belgium, 17 - 23 September 1975. Universa Press, Wetteren, 1: 381 - 390
Dunstan, W.M. & Menzel, D.W. 1971. Continuous culture of natural populations of phytoplankton in dilute treated sewage effluent. Limnol. Oceanogr. 16: 623 - 632
Griffith, G.W., Murphy Kenslow, M.A. & Rose, L.A. 1973. A mass culture method for Tetraselmis sp. - a promising food for larval crustaceans. In: J. W. Avault. Jr. (ed) Proceedings of the 4th Annual Workshop of the World Mariculture Society. Louisiana State University, Baton Rouge: 289 - 294
Guillard, R.L. 1975. Culture of phytoplankton for feeding marine invertebrates, p. 29 - 60. In: P.B. Smith (ed) Culture of Marine Invertebrates. Plenum Press, New York.
Harrison, P.J., Waters, R.E. & Taylor, F.J.R. 1980. A broad spectrum artificial seawater medium for coastal and open ocean phytoplankton. J. Phycol. 16: 28 - 35
Helm M.M., Laing, I. & Jones, E. 1979. The development of a 200 l algal culture vessel at Conwy. Fish. Res. Tech. Rep., MAFF Direct. Fish. Res., Lowestoft, 53 (1): 1 - 7
Kranck, K. & Milligan, T. 1979. The Use of the Coulter Counter in Studies of Particle Size Distributions in Aquatic Environments. Bedford Inst. Oceanography. Dartmouth, Nova Scotia. Rep. Ser. BI-R-79-7: 48 pp.
Laing, I. 1979. Recommended procedures for the culture of Chaetoceros calcitrans. Fish. Res. Tech. Rep., MAFF Direct. Fish. Res., Lowestoft, 53 (2): 8 - 12
Laing, I. 1985. Growth response of Chaetoceros calcitrans (Bacillariophyceae) in batch culture to a range of initial silica concentrations. Mar. Biol., 85: 37 - 41
Laing, I. 1987. The use of artificial diets in rearing bivalve spat. Aquaculture, 65: 243 - 249
Laing, I. 1990. Nutritional value of dried algae diets for larvae of Manila clam, Tapes philippinarum. J. Mar. Biol. Assoc., UK, 70: 1 - 12
Laing, I. & Ayala, F. 1987. Commercial mass culture techniques for producing microalgae. p 447 - 477. In: Akatsuka (ed) Introduction to Applied Phycology. Academic Publishing, The Hague, The Netherlands
Langdon, C.J. & Waldock, M.J. 1981. The effect of algal and artificial diets on the growth and fatty acid composition of Crassostrea gigas spat. J. Mar. Biol. Assoc. UK, 61: 431 - 448
Laing, I. & Helm, M.M. 1981. Cost effective culture of marine unicellular algae. In: F. Vogt (Ed) Energy Conservation and Use of Renewable Energies in the Bio-Industries. Pergammon Press, Oxford: 247 - 259
Laing, I. & Helm, M.M. 1981. Factors affecting the semi-continuous production of Tetraselmis suecica (Kylin) Butch. in 200 l vessels. Aquaculture, 22: 137 - 148
Laing, I. & Jones, E. 1983. Large scale turbidostat culture of marine microalgae. Aquacultural Engineering, 2: 203 - 212
Laing, I. & Jones, E. 1988. A turbidostat vessel for the continuous culture of marine microalgae. Aquacultural Engineering, 7: 89 - 96
Mann, R. & Ryther, J.H. 1977. Growth of six species of bivalve molluscs in a waste recycling aquaculture system. Aquaculture, 11: 231 - 245
Roels, O.A., Haines, K.C. & Sunderlin, J.B. 1975. The potential yield of artificial upwelling mariculture. In: G. Persoone and E. Jaspers (eds) Proceedings of the 10th European Symposium on Marine Biology, Ostend, Belgium, 17 - 23 September 1975. Universa Press, Wetteren, 1: 381 - 390
Sheldon, R.W. & Parsons, T.R. 1967. A Practical Manual for the Use of the Coulter Counter in Marine Science. Coulter Electronics, Toronto, Ontario: 66 pp.
Spencer, B.E. 1988. Growth and filtration of juvenile oysters in experimental outdoor pumped upwelling systems. Aquaculture, 75: 139 - 158
Stein, J.R. 1973. Handbook of Phycological Methods: Culture Methods and Growth Measurements. Cambridge University Press, Cambridge, England: 448 pp.
Trotta, P. 1981. A simple and inexpensive system for continuous monoxenic mass culture of marine microalgae. Aquaculture, 22: 383 - 387
Ukeles, R. 1973. Continuous culture - a method for the production of unicellular algal foods. In: J.R. Stein (ed), Handbook of Phycological Methods, Culture Methods and Growth Measurements. Cambridge University Press, Cambridge: 233 - 254
Ukeles, R. 1973. Cultivation of plants. In: O. Kinne (ed.), Marine Ecology, John Wiley and Sons, New York, NY, Cultivation, 3 (1): 367 - 466
Walne, P.R. 1970. Studies on the food value of nineteen genera of algae to juvenile bivalves of the genera Ostrea, Crassostrea, Mercenaria, and Mytilus. Fishery Invest., Lond., Ser. 2, 26 (5): 1 - 62
Webb, K.L. & Chu, F.-L.E. 1983. Phytoplankton as a source of food for bivalve larvae. In: (eds: Pruder, G.D., Langdon, C. & Conklin, D.) Proceedings of the 2nd International Conference on Aquaculture Nutrition: Biochemical and Physiological Approaches to Shellfish Nutrition, October 1981, Rehoboth Beach, Delaware. Louisiana State University Press, Baton Rouge: 272 - 291
Whyte, J.N.C. 1987. Biochemical composition and energy content of six species of phytoplankton used in mariculture of bivalves. Aquaculture, 60: 231 - 241
Wisley, B. & Purday, C. 1961. An algal mass culture unit for feeding marine invertebrate larvae. Tech. Pap. Div. Fish. Oceanogr. CSIRO, Australia, 12: 2 - 12