First Law of Thermodynamics
Last Updated :
08 Apr, 2024
First Law of Thermodynamics adaptation of the Law of Conservation of Energy differentiates between three types of energy transfer: Heat, Thermodynamic Work, and Energy associated with matter transfer. It also relates each type of energy transfer to a property of a body’s Internal Energy.
The First Law of Thermodynamics states that energy cannot be created or destroyed however, it can be transferred from one form to another. Also, according to the first law of thermodynamics, Heat is a form of energy and the thermodynamic processes (like Isothermal, Isochoric, Adiabatic, Isothermal, and Quasi-Static Processes.) obey the Law of Conservation of Energy.
What is the First Law of Thermodynamics?
First Law of Thermodynamics states that the total energy of an isolated system is constant. Energy can be transformed from one form to another, but can neither be created nor destroyed.
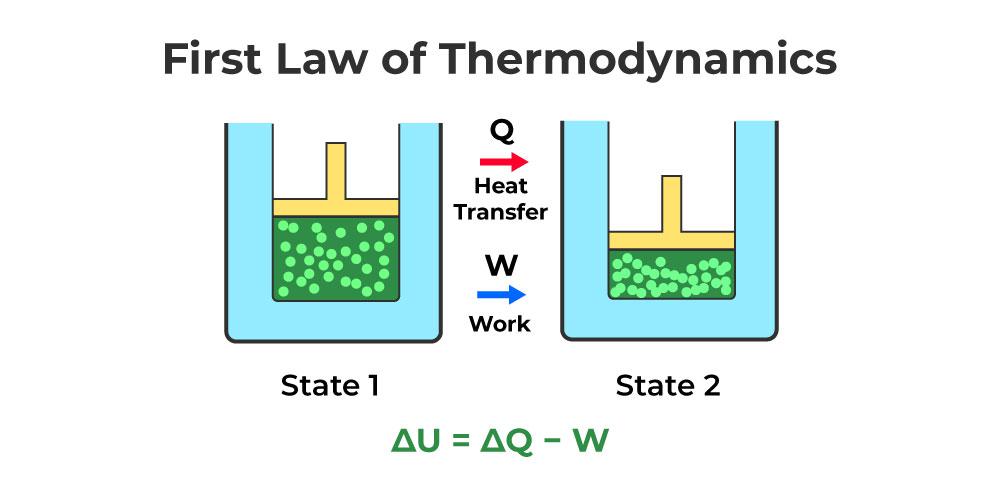
Internal energy is a state variable in a thermodynamic system that is in equilibrium. The internal energy difference between the two systems is equal to heat transfer into the system minus work done by the system.
According to the First Law of Thermodynamics, the universe’s energy does not change. It can be transferred between the system and the surroundings, but it cannot be produced or destroyed. The law is primarily concerned with energy states as a result of work and heat transmission.
We may use the popular example of a heat engine to help you grasp the meaning of the First Law. Thermal energy is transformed into mechanical energy in a Heat engine, and the process is also reversed. The majority of heat engines are classified as open systems. A heat engine’s primary working concept is to take advantage of the many interactions between heat, pressure, and volume of a working fluid, which is generally a gas. It’s not uncommon for gas to turn into a liquid and then back into a gas.
According to this law, some heat supplied to the system is used to change the internal energy, while the remaining is used by the system to perform work. The mathematical expression of the first law of thermodynamics is given by:
ΔQ = ΔU + ΔW
Where
- ΔU is the change in internal energy of the system,
- ΔW is the work done by the system, &
- ΔQ is the heat supplied to the system.
Limitations of First Law of Thermodynamics
- The first law of thermodynamics has a limitation in that it states nothing about the direction of heat flow.
- It is not feasible to reverse the procedure. In actuality, the heat does not entirely convert to labor. We could move ships across the ocean by extracting heat from the ocean’s water if it had been feasible to turn all of the heat into work.
- It makes no distinction between whether the process is spontaneous or not.
Perpetual Motion Machine of First Kind (PMM1)
It is impossible to build a machine that can do mechanical work indefinitely without spending any energy. The perpetual motion machine of the first type is a hypothetical device like this. These machines contradict the first rule of thermodynamics and do not exist in the actual world.
First Law of Thermodynamics for a Closed System
The product of the pressure applied and the change in volume that happens as a result of the applied pressure is the work done for a closed system:
W = – P ΔV
Where
- P denotes the system’s constant external pressure, and
- V denotes the volume change.
This is referred to as Pressure-Volume work.
The internal energy of a system rises or falls in response to work interactions that occur across its limits. When work is done on the system, the internal energy increases, but it decreases when work is done by the system. Any heat exchange between the system and its surroundings alters the system’s internal energy. However, the total change in internal energy is always zero since energy remains constant (according to the first rule of thermodynamics). If the system loses energy, it is absorbed by the surroundings. If energy is absorbed into a system, the energy must have been released by the environment:
ΔUsystem = −ΔUsurroundings
Where
- ΔUsystem is the change in the total internal energy of the system, and
- ΔUsurroundings is the change in the total energy of the surrounding.
Related Articles:
Solved Examples on First Law of Thermodynamics
Example 1: Find out the internal energy of a system that has constant volume and the heat around the system is increased by 30 J.
Solution:
Given that,
Heat Transfer, ΔQ = 30 J
For constant volume, ΔV = 0
W = P ΔV = 0
The formula for internal energy is given as:
ΔU = ΔQ – W
⇒ ΔU = 30 J – 0
⇒ ΔU = 30 J
Hence, the change in internal energy of the system is 30 J.
Example 2: Calculate the change in the internal energy of the system if 2000 J of heat is added to a system and a work of 1500 J is done.
Solution:
Given that,
Heat added to a system, ΔQ = 2000 J
Work done on the system, W = 1500 J
The formula for internal energy is given as:
ΔU = ΔQ – W
⇒ ΔU = 2000 J – 1500 J
⇒ ΔU = 500 J
Hence, the change in internal energy of the system is 500 J.
Example 3: A gas in a closed container is heated with 20 J of energy, causing the lid of the container to rise 3 m with 4 N of force. What is the total change in energy of the system?
Solution:
Given that,
Heat supplied to the container, ΔQ = 20 J
Rise in lid of the container, Δx = 3 m
Force applied on the container, F = 4 N
We are not given a value for work, but we can solve for it using the force and distance. Work is the product of force and displacement.
W = F Δx
⇒ W = 4 N × 3 m
⇒ W = 12 J
The formula for internal energy is given as:
ΔU = ΔQ – W
⇒ ΔU = 20 J – 12 J
⇒ ΔU = 8 J
Hence, the change in internal energy of the system is 8 J.
Example 4: Determine the change in the internal energy of the system when gas in a cylinder is fitted with a frictionless piston expands against a constant external pressure of 1 atm from a volume of 2 liters to a volume of 5 liters. So it absorbs 100 J of thermal energy from its surroundings.
Answer:
Given that,
Q = 100 J
V1 = 2 L
V2 = 5 L
Then, according to the formula:
ΔU = Q – PΔV
⇒ ΔU = Q – P(V2 – V1)
Therefore,
ΔU = Q – P(V2 – V1)
⇒ ΔU = 100 J – 1 (5 – 2) 101.33 J
⇒ ΔU = -203.99 J
FAQs on First Law of Thermodynamics
What is the First Law of Thermodynamics?
The first law of thermodynamics deals with work done and heat energy supplied or removed from a system. The First Law of Thermodynamics states that the total energy of an isolated system is constant. Energy can be transformed from one form to another, but can neither be created nor destroyed.
State the Mathematical Form of the First Law of Thermodynamics.
The mathematical expression of the first law of thermodynamics is given by,
ΔQ = ΔU + ΔW
where
- ΔU is the change in internal energy of the system,
- ΔW is the work done by the system, &
- ΔQ is the heat supplied to the system.
What is the Significance of the First Law of Thermodynamics to the Environment?
Energy cannot be generated or destroyed, according to the first law; it can only be changed from one form to another. All living species on Earth rely on the sun for their energy.
Photosynthesis is the process through which plants transform solar energy into chemical energy. These energies are not returned to the solar system by the plants; instead, they are passed on to herbivores that eat green vegetation.
Carnivores use some energy gained by herbivores, while some energy obtained by herbivores is passed to decomposers when the herbivores die.
Name the Law of Conservation which is followed by the First Law of Thermodynamics.
The Law of Conservation of Energy is followed by the First Law of Thermodynamics.
What is an Isolated System?
An Isolated System is an open system in which the exchange of both energy and matter with its surroundings takes place.
What are the Applications of the First Law of Thermodynamics?
The First Law of Thermodynamics exhibit a wide variety of applications in the field of Physics. Here are the Applications of the First Law of Thermodynamics: In Engines, Refrigerator, Thermal Power Plants, etc.
Name those physical parameters of matter for which the First Law of Thermodynamics provides the relation.
The First law of thermodynamics furnishes the relationship between heat, work, and the internal energy of the system i.e. the properties of the system.