This article, the second in a two-part series on cardiomyopathies, discusses diagnostic interventions, management options and implications for nursing practice
Abstract
Cardiomyopathies are diseases characterised by structural and functional abnormalities of the myocardium. There are various types and subtypes, which means that tests, treatments and patient advice will differ accordingly. The first article in this two-part series on cardiomyopathies described their classification, pathophysiology and clinical presentation. This second article covers diagnostic investigations, management options and implications for practice. Nurses have a central role to play in informing, advising and supporting patients.
Citation: Jarvis S (2019) Cardiomyopathies 2: diagnosis, treatment and practice implications. Nursing Times [online]; 115: 8, 29-33.
Author: Selina Jarvis is research nurse and former Mary Seacole development scholar, Kingston University and St George’s, University of London, and King’s Health Partners, Guy’s and St Thomas’ NHS Foundation Trust.
- This article has been double-blind peer reviewed
- Scroll down to read the article or download a print-friendly PDF here (if the PDF fails to fully download please try again using a different browser)
- Read part 1 of this series here
Introduction
Cardiomyopathies are a mixed group of disorders affecting the myocardium that present with a variety of symptoms and signs caused by either structural or functional abnormalities. Part 1 in this two-part series on cardiomyopathies discussed classification, clinical signs and key types. In part 2, the focus is on diagnostic investigations, treatment options and implications for nursing practice.
Investigations
Given the complexity of cardiomyopathies (see part 1), several different clinical, laboratory and imaging approaches may be needed (Fig 1). After taking a detailed medical history and conducting a thorough clinical examination, the clinician will usually perform investigations to confirm certain symptoms and signs that are associated with a particular cardiomyopathy.
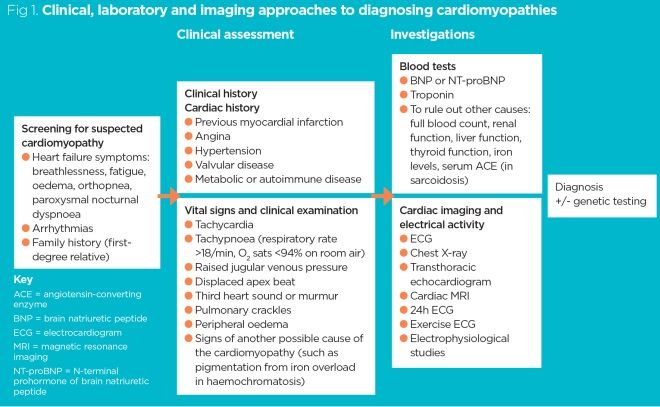
Investigations into suspected cardiomyopathy generally rely on structural imaging techniques such as echocardiography and cardiac magnetic resonance imaging (MRI), but tests that are easier to perform – such as 12-lead electrocardiograms (ECGs), blood tests and chest radiographs – can be used to support the diagnosis. In certain circumstances, 24-hour ECGs and exercise ECGs can be useful.
Some cardiomyopathies can be inherited, so a taking a detailed family history is crucial. If diagnostic criteria for inherited cardiomyopathy are met, genetic testing may be performed to confirm the diagnosis.
Patients with suspected cardiomyopathy may present with signs and symptoms, but they may also be asymptomatic (see part 1). Some people undergo investigations because they have a first-degree relative with an inherited cardiomyopathy; these investigations will detect whether they have also inherited it and whether, as a consequence, they may be at risk of sudden cardiac death (SCD) (see part 1).
Electrocardiogram
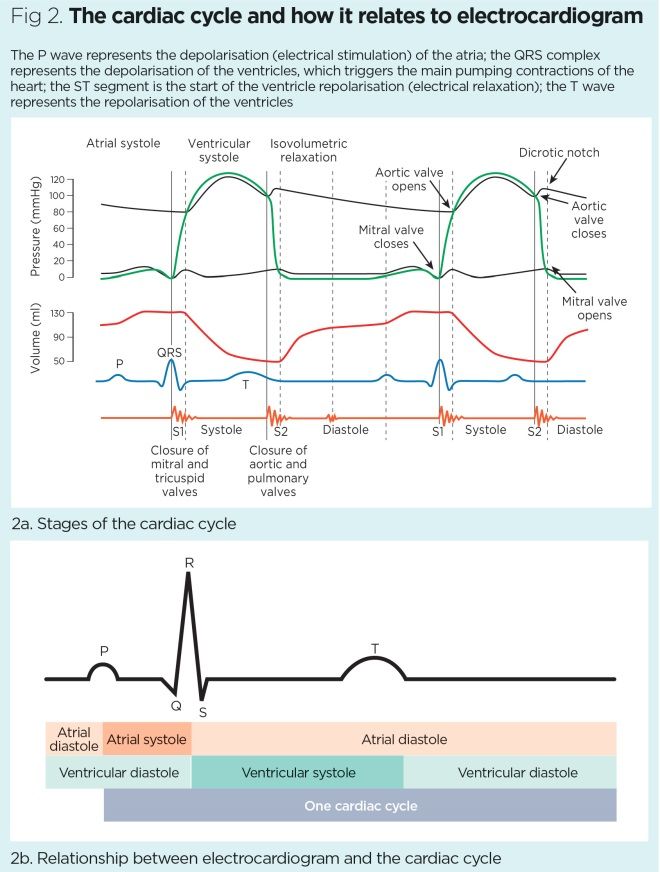
An ECG is not diagnostic but can show features suggestive of an underlying cardiomyopathy and any associated arrhythmias. Fig 2 shows the relationship between an ECG and the cardiac cycle.
Dilated cardiomyopathy
In dilated cardiomyopathy (DCM) – the most common type – the ECG may display non-specific features of an increased left ventricular muscle mass suggestive of left ventricular hypertrophy (LVH), pathological Q waves and poor R wave progression, with some abnormalities affecting the T wave and ST segment. Sometimes, electrical conduction down the ventricles may be disrupted and left or right bundle branch block may ensue, in which case the ECG may show a widened QRS complex; this carries with it a worse prognosis. Sinus tachycardia, or even ventricular tachycardia, may also exist (Sisakian, 2014).
Hypertrophic cardiomyopathy
In hypertrophic cardiomyopathy (HCM), the ECG may display features of LVH and there may be high voltage complexes, left axis deviation and P wave abnormalities. Additionally, around 20-30% of patients with HCM have atrial fibrillation which, due to the fibrillating atria, may translate on the ECG as irregular rhythm strips and a loss of P waves.
Restrictive cardiomyopathy
In restrictive cardiomyopathy (RCM), patients have enlargement of both atria (biatrial enlargement) due to the stiffness of the heart. On the ECG, there may be large P waves and changes suggestive of left ventricular hypertrophy (large R waves).
Arrhythmogenic cardiomyopathy
In arrhythmogenic cardiomyopathy (ACM) there may be ECG abnormalities that reflect defective conduction through the diseased right ventricular myocardium. Ventricular tachycardias or ectopic beats, as well as bundle branch block and inverted T waves in inferolateral leads, may be seen (McKenna, 2019; Corrado et al, 2017). More specifically, an epsilon wave – a small-amplitude signal seen at the end of the QRS wave and the start of the T wave – may be seen, albeit infrequently (Rapezzi et al, 2013).
Cardiac biochemistry
From a blood biochemistry perspective, there is a hormone that acts as a cardiac marker and can help diagnose cardiomyopathies: brain natriuretic peptide (BNP) (Jarvis and Saman, 2017). BNP is responsible for fluid and sodium loss in the urine. It is released by the heart when the ventricles are stretched, which occurs in congestive heart failure. BNP has some diagnostic utility and a high negative predictive value (97%); it is, therefore, helpful in ruling out heart failure.
In a known cardiomyopathy, BNP may have some prognostic relevance. In HCM it can be useful in conjunction with other tests. In RCM and ACM, although there may be an increase in serum levels of BNP, this only suggests – but cannot diagnose – a failing heart.
Other useful cardiac markers include troponin I and troponin T: elevated levels of troponins (Troponin I or T) indicate possible damage to cardiomyocytes. Troponins may be useful in the context of HCM, in which higher levels are associated with left ventricular thickness and worsening diastolic dysfunction. Elevated levels of troponins are also associated with:
- A higher risk of hospital admission for heart failure;
- A higher risk of death from cardiovascular disease;
- Ventricular tachycardia;
- Progression of heart failure symptoms over the next four years (Kubo et al, 2013).
Cardiac imaging
Cardiac imaging is an important part of the diagnostic work-up of cardiomyopathies. Initially, a plain chest radiograph is usually taken, which can confirm the presence of cardiomegaly – for instance in DCM. Depending on the degree of HF, there may be signs of pulmonary venous congestion, such as upper lobe diversion or Kerley B lines – these are horizontal subpleural lines measuring 1-2cm found near the costophrenic angles of the lungs and may be indicative of fluid in the lung tissue.
Imaging modalities used to diagnose cardiomyopathy include 2D transthoracic echocardiography and cardiac MRI (cMRI). Both provide structural and haemodynamic information; while 2D echocardiography is absolutely required and usually diagnostic, cMRI – although not used routinely – is necessary to diagnose certain cardiomyopathies using a contrast agent (for example, gadolinium). It can help differentiate between ischaemic and non-ischaemic causes, and identify scarring of cardiac tissues.
Dilated cardiomyopathy
Echocardiography for DCM is often diagnostic, showing characteristic enlargement of the left ventricle and, sometimes, the right ventricle. There may also be atrial dilatation (possibly associated with a thrombus). Using the ‘M’ mode of echocardiography (which provides dynamic time-motion imaging) can also help assess:
- The motion of the heart valves;
- Chamber sizes;
- Ventricle thickness and function.
In DCM, there are diffusely hypokinetic walls and the impaired systolic function of the ventricle can be visualised (Sisakian, 2014). Doppler measurements may demonstrate functional mitral or tricuspid regurgitation with backflow of the blood across the valves.
Hypertrophic cardiomyopathy
In HCM, left ventricular outflow tract obstruction (LVOT) – an important mechanism responsible for causing symptoms of heart failure – is a highly visible feature on imaging such as echocardiography or cMRI. Imaging may also reveal some asymmetry of the septum between the ventricles and hypertrophy. Additionally, there may be impaired motion of the anterior leaflet of the mitral valve.
The diagnostic criteria for HCM can be demonstrated with echocardiography or cMRI, which will show that the left ventricular wall is thickened (measuring >15mm thickness in one or more of the myocardial segments) (Sisakian, 2014).
Restrictive cardiomyopathy
In RCM, which is most commonly caused by infiltrative disease, the left ventricular walls may be thickened, with features of moderate-to-severe left ventricular dysfunction. There may be biatrial enlargement (due to backflow of pressure in the heart) and reduced isovolumetric relaxation (Sisakian, 2014). With cMRI, there may also be specific signs of the underlying cause, such as ‘granular sparkling’ of the myocardium in amyloidosis and myocardial calcifications or endocardial thickening in endomyocardial fibrosis (Sisakian, 2014).
Arrythmogenic cardiomyopathy
In suspected ACM, echocardiography and cMRI should be undertaken in all patients. Imaging will show abnormalities of the right ventricle (dilatation dysfunction) and some regional wall motion abnormalities. The left ventricle and septum may also be involved, but this happens less often. cMRI with gadolinium is the preferred imaging modality to visualise right ventricular abnormalities and identify the fibrofatty myocardial scarring that is characteristic of ACM (Corrado et al, 2017).
Treatment
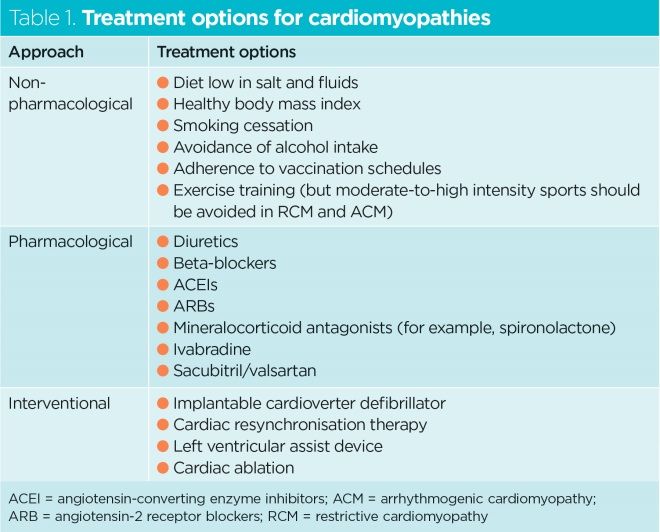
Treatment options for cardiomyopathies are outlined in Table 1 and detailed below.
Non-pharmacological approaches
Non-pharmacological approaches are important in the management of cardiomyopathies. They include patient self-care behaviours such as restricting salt and fluids, avoiding smoking and alcohol, maintaining a healthy body mass index and adhering to vaccination schedules. There is evidence that exercise training can improve quality of life in patients with DCM (Weintraub et al, 2017).
In RCM, and even more so in ACM, treatment aims to reduce the risk of SCD. Practising moderate-to-high-intensity, competitive recreational sports increases patients’ risk of SCD, so they should be advised to avoid doing so (Corrado et al, 2017).
Multidisciplinary team involvement and education on heart failure will be key to help patients make, and adhere to, these lifestyle changes.
Pharmacological approaches
Pharmacological treatment can prevent progression of the disease. In patients with acute deterioration of cardiomyopathy causing heart failure, management focuses on treating the fluid overload (which is likely causing pulmonary and peripheral oedema) with intravenous loop diuretics such as furosemide. Some patients may also need:
- Sublingual or intravenous glyceryl trinitrate (to help relieve the symptoms of pulmonary congestion);
- Non-invasive ventilation in the form of continuous positive airway pressure, which can help improve the pulmonary oedema (Weintraub et al, 2017).
Pulmonary oedema can be life threatening if not treated promptly; patients with pulmonary oedema often present to the acute setting.
In the long-term care perspective, there are slight differences in the medications used to treat each type of cardiomyopathy.
Dilated cardiomyopathy
In DCM, the cornerstones of pharmacological therapy are angiotensin converting enzyme inhibitors (ACEIs), angiotensin-2 receptor blockers (ARBs), beta-blockers and mineralocorticoid antagonists, such as spironolactone and, sometimes, ivabradine. There is good evidence that these medications improve survival in patients with heart failure; it is crucial they are taken daily.
New treatments such as the combination of sacubitril and valsartan may be considered to treat cardiomyopathy in which there is reduced ejection fraction. Sacubitril contains a neprilysin (NEP) inhibitor; valsartan is an ARB class of drug.
NEP is an enzyme that metabolises different peptides, including BNP; if it is prevented from working (using a neprilysin inhibitor), there is less breakdown of BNP. This drug therefore promotes diuresis and vasodilation, and has several other beneficial effects.
The use of sacubitril-valsartan has produced evidence of lower cardiovascular mortality and lower rates of hospitalisation for exacerbations compared with enalapril (Jhund et al, 2015). Sacubitril-valsartan has been incorporated into US guidance on the management of HF (Yancy et al, 2016).
Hypertrophic cardiomyopathy
The pharmacological management of HCM is less clear, but there is no doubt that beta-blockers are important, as they reduce physiological outflow obstruction, angina, dyspnoea and the risk of ventricular arrhythmias associated with HCM. The negative chronotropic effect (reduction in heart rate) and negative inotropic effect (reduction in the force generated by the heart) of beta-blockers can ultimately lead to more diastolic filling time for the heart. Although is it is not clear whether beta-blockers have life-prolonging benefits, they are recommended in the standard management of HCM (Houston and Stevens, 2015).
There is some evidence that calcium channel blockers such as verapamil and diltiazem can also improve filling and reduced outflow gradient but, in cases of severe outflow obstruction or severe HF, caution is required when using them (Houston and Stevens, 2015).
ACEIs and ARBs are not routinely used in patients with HCM but valsartan may suppress the production of collagen that causes fibrosis in HCM. More trials are needed in this area (Kawano et al, 2005).
Restricted cardiomyopathy
In RCM, symptomatic treatment is advocated as there is restriction and stiffening of the right and left ventricles leading to diastolic dysfunction and fluid overload (causing peripheral and pulmonary oedema). Diuretics are a key element of treatment, as they reduce fluid volume and congestion symptoms. Careful dosing is important as overtreatment with diuretics can lead to low blood pressure and dizziness.
As the ventricles are stiffened in the context of exercise, the heart is very dependent on an increase in heart rate to maintain blood flow and cardiac output. As such, beta-blockers or calcium channel blockers – which have negative chronotropic effects – are not recommended in RCM.
Finally, in patients who have RCM with atrial enlargement, anticoagulation may be necessary to lower the risk of arrhythmias and thromboembolism.
In RCM caused, for example, by haemochromatosis or sarcoidosis (see part 1), the underlying condition might warrant specific treatments to prevent progression of the cardiomyopathy (Mogensen, 2019).
Arrhythmogenic cardiomyopathy
In ACM the aim of treatment is to lower the risk of SCD and reduce heart failure and arrhythmias. There is limited data on the use of beta-blockers in this type of cardiomyopathy, but they are recommended to prevent arrhythmias and reduce the stress on the wall of the right ventricle. Patients who have an episode of sustained ventricular tachycardia or ventricular fibrillation may benefit from cardiac devices (implantable cardioverter defibrillators [ICDs]) or require catheter electrical ablation (Corrado et al, 2017).
Interventional approaches
Cardiac devices include ICDs and cardiac resynchronisation therapy (CRT). They can decrease rates of SCD, and are crucial in the management of certain cardiomyopathies. An ICD records heart activity and treats dangerous ventricular arrhythmias by delivering an electric shock. With CRT, the aim is to retrain the heart and relieve symptoms by synchronising left ventricular contraction (Knight, 2019). Cardiac devices will often be used to treat patients with cardiomyopathies associated with ventricular tachycardias or bradycardias.
Patients with DCM stemming from non-ischaemic reasons will have little to gain from an ICD. However, ICDs can be a treatment option for people with genetic conditions such as ACM and in some with HCM, where there is a high risk of SCD. The ICD may also slow down the rate of disease progression.
Advanced cardiac device therapies, as well as cardiac transplantation, may be beneficial for certain patients. Left ventricular assist devices (LVADs) may help certain patients (for example, those with RCM) or serve as a ‘bridge’ while patients await a heart transplant. There have been landmark clinical trials on the use LVADs: an early trial showed a 48% reduction in all-cause mortality and increased survival at one and two years in patients fitted with an LVAD (Rose et al, 2001).
Implications for practice
Patients with a cardiomyopathy should have their vital signs monitored, and need to be encouraged to gain a good understanding of their condition, medications and possible side-effects. They must be encouraged to adopt self-care behaviours and make lifestyle changes. Nursing staff have a vital role in this process, as they can:
- Counsel patients on diet, smoking, alcohol consumption, body mass index and vaccination;
- Increase patients’ awareness of factors that might exacerbate their condition and lead to acute heart failure;
- Reiterate advice about avoiding moderate-to-high-intensity sport to patients with HCM or ACM.
When patients have a cardiac device, particularly an ICD (which is increasingly common), nurses need to:
- Be aware of the presence of the device;
- Ask patients when it last delivered a shock;
- Ask patients whether they have received inappropriate shocks.
Box 1 highlights the maintenance needs and long-term complications of ICDs.
Box 1. Implantable cardioverter defibrillators: maintenance and risks
Maintenance needs
- ICDs are usually powered by lithium batteries, which last from 6-12 years
- Patients with ICDs require monitoring every 3-6 months for life
- Modern ICDs continuously record the electrical activity of the heart, and record the date and time of each episode when the ICD fires to deliver a shock
- Patients experiencing a shock from their ICD need to be advised to tell their doctor
- Most arrhythmias require only one shock to return the heart rhythm to normal, so patients who have frequent shocks or clusters of shocks must be evaluated urgently
- MRI should be avoided in patients with ICDs
Long-term risks
- Infection or erosion: ICD needs to be removed
- Lead failure: mechanical stresses on the leads can lead to breakage of the wires within the leads or in the insulation
- Inappropriate detection of an arrhythmia and subsequent delivery of a shock: ICD needs urgent investigation
- Premature battery depletion or device failure: ICD needs urgent investigation
ICD = implantable cardioverter defibrillator; MRI = magnetic resonance imaging
Source: Adapted from Knight (2019)
Conclusion
Cardiomyopathies are a heterogeneous group of cardiac muscle disorders. As each type has specific features, diagnostic interventions, treatment options and patient advice will differ. Cardiac imaging is crucial in the diagnostic work-up and patients should be referred for cMRI when needed.
In certain types of cardiomyopathies, there is good evidence to support the use of pharmacological approaches and cardiac devices. Most importantly, professional societies stress the importance of self-care behaviours and lifestyle changes – an area in which nursing staff have a key role.
Key points
- To correctly diagnose cardiomyopathies, several investigations may be needed
- The diagnostic investigations for cardiomyopathies include electrocardiograms, biochemistry and imaging
- Treatments encompass pharmacological and non-pharmacological approaches
- The use of cardiac devices in patients who have cardiomyopathies is becoming more common
- The role of nurses in encouraging patients to adopt self-care behaviours is key
Houston BA, Stevens GR (2015) Hypertrophic cardiomyopathy: a review. Clinical Medical Insights. Cardiology; 8: Suppl 1, 53-65.
Jarvis S, Saman S (2017) Heart failure 2: treatment options and long-term management. Nursing Times [online]; 113: 9, 55-58.
Jhund PS et al (2015) Efficacy and safety of LCZ696 (sacubitril-valsartan) according to age: insights from PARADIGM-HF. European Heart Journal; 36: 38, 2576-2584.
Kawano H et al (2005) Valsartan decreases type I collagen synthesis in patients with hypertrophic cardiomyopathy. Circulation Journal; 69: 10, 1244-1248.
Knight BP (2019) Patient education: implantable cardioverter-defibrillators (Beyond the Basics).
Kubo T et al (2013) Significance of high-sensitivity cardiac troponin T in hypertrophic cardiomyopathy. Journal of the American College of Cardiology; 62: 14, 1252-1259.
McKenna WJ (2019) Arrhythmogenic right ventricular cardiomyopathy: diagnostic evaluation and diagnosis.
Mogensen J (2019) Restrictive cardiomyopathy. In: Camm AJ et al (eds) The ESC Textbook of Cardiovascular Medicine. Oxford: Oxford University Press.
Rapezzi C et al (2013) Diagnostic work-up in cardiomyopathies: bridging the gap between clinical phenotypes and final diagnosis. A position statement from the ESC Working Group on Myocardial and Pericardial Diseases. European Heart Journal; 34: 19, 1448-1458.
Rose EA et al (2001) Long-term use of a left ventricular assist device for end-stage heart failure. New England Journal of Medicine; 345: 20, 1435-1443.
Sisakian H (2014) Cardiomyopathies: evolution of pathogenesis concepts and potential for new therapies. World Journal of Cardiology; 6: 6, 478-494.
Weintraub RG et al (2017) Dilated cardiomyopathy. Lancet; 390: 10092, 400-414.
Yancy CW et al (2016) 2016 ACC/AHA/HFSA focused update on new pharmacological therapy for heart failure: an update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Journal of the American College of Cardiology; 68: 13, 1476-1488.
