This article, the first in a two-part series on pulmonary administration of medicines, summarises the use of inhaled therapies and describes the procedure for teaching patients to use pressurised metered dose inhalers with and without a spacer
Abstract
Inhaled medications are the mainstay of pharmacological treatment for people with chronic obstructive pulmonary disease and asthma. Correct inhaler technique and appropriate delivery devices are essential for optimal treatment. This first article in a two-part series provides an introduction to using inhaled therapies and describes the procedure for teaching patients to use pressurised metered-dose inhalers with and without a spacer. Part 2 will describe the procedure for the use of dry-powder inhalers and nebulisers for medicines administration.
Citation: Rickards E, Proctor J (2021) Pulmonary administration medicines 1: procedure for use of PMD inhalers. Nursing Times [online]; 117: 1, 18-21.
Authors: Emma Rickards is a respiratory nurse consultant, Liverpool Heart and Chest Hospital NHS Foundation Trust and Knowsley Community Respiratory Service; Jaclyn Proctor is a respiratory advanced nurse practitioner, Warrington Hospital.
- This article has been double-blind peer reviewed
- Scroll down to read the article or download a print-friendly PDF here (if the PDF fails to fully download please try again using a different browser)
- Read part 2 of this series here
Introduction
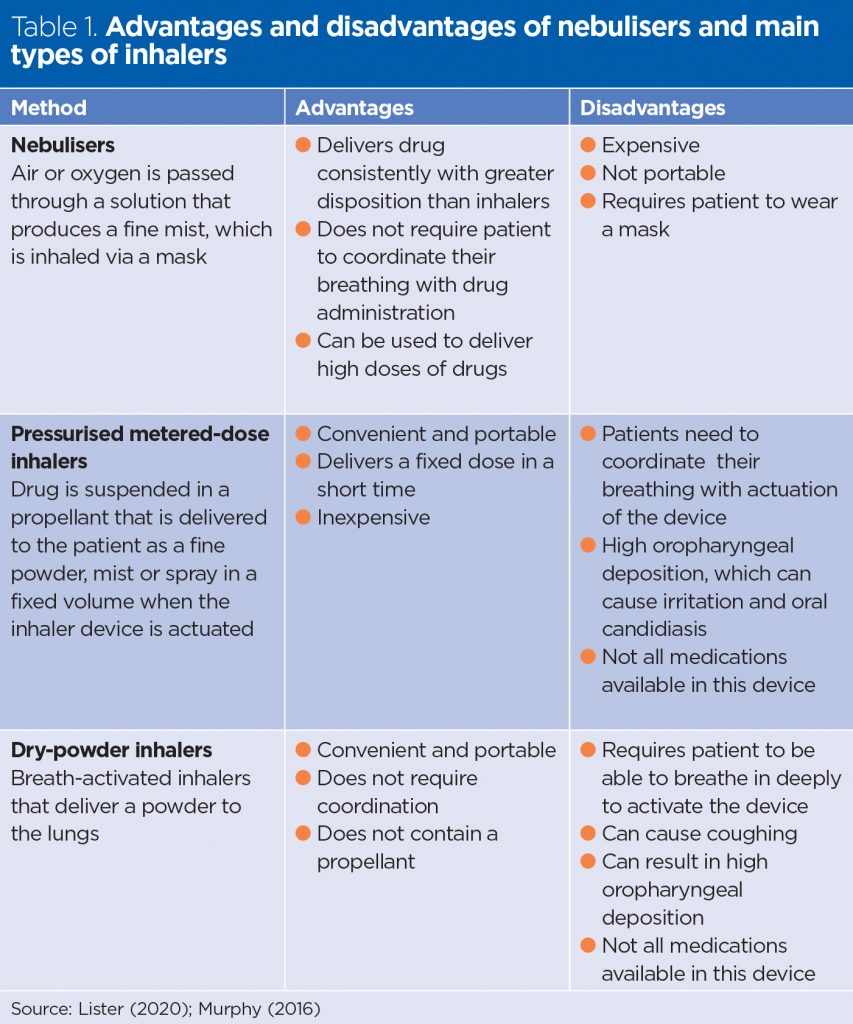
The lungs are vascular organs with a large surface area. Drugs can be delivered into the respiratory tract to achieve a local effect and minimise systemic side-effects, for example, bronchodilators and corticosteroids in the treatment of asthma – this is known as pulmonary administration. Drugs such as anaesthetics may also be administered via this route (Lister et al, 2020). Drugs commonly used in the treatment of respiratory disease are usually delivered in an aerosol form by pressurised metered-dose inhalers (pMDI), dry-powder inhalers (DPI) and nebulisers.
Benefits of aerosol therapies
Aerosol therapy, when used correctly, provides a fast, effective route for treating lung disorders because the drug is delivered to the site of action (Tena and Clarà, 2012). Its effectiveness depends on both the amount of drug that successfully passes beyond the oropharyngeal region and where it is deposited in the lungs.
Effective deposition of inhaled medication to the lungs aims to:
- Reduce symptoms such as breathlessness;
- Improve exercise tolerance, quality of life and overall health status;
- Prevent disease progression;
- Prevent and treat exacerbations;
- Reduce mortality (Global Initiative for Asthma, 2020; Global Initiative for Chronic Obstructive Lung Disease, 2020).
In the management of asthma, inhaled therapy is primarily used to achieve good symptom control. This involves the use of inhaled medicines to suppress the inflammatory response in the airways that causes symptoms, thereby minimising the future risk of exacerbations (GINA, 2020).
The advantages and disadvantages of different devices are outlined in Table 1.
Inhaler technique
The correct use of inhalers will avoid treatment failure, improve symptom control and reduce the risk of adverse effects, such as increased exacerbations of symptoms and premature escalation of treatment (Scullion, 2017). There are many different inhaler types. In the UK, pMDIs are the most commonly used delivery system for treating asthma, chronic obstructive pulmonary disease (COPD) and other respiratory diseases (Murphy, 2016).
A large observational study by Melani et al (2011), which included 1,664 patients with asthma (42%) and COPD (52%) who were using pMDIs (843) and/or DPIs (1,113) at home, demonstrated that poor inhaler technique is associated with worsening outcomes. DPIs require less coordination to use than pMDIs and this can improve the delivery of the drug to the lungs. However, patients who use a DPI need to be able to breathe deeply to activate the device, which is not possible for all patients. Overall, despite changes in inhaler technology, inhaler technique for both pMDI and DPI has not improved over 40 years (Sanchis et al, 2016).
The UK Inhaler Group (UKIG) (2019) recommends that health professionals who are starting a patient on an inhaler device should know how to use it and be competent in teaching the required technique to ensure appropriate administration of the drug. It is also important to involve the patient when selecting a device and avoid changing it, as this may require them to learn a new technique.
Additionally, reviewing inhaler technique should be an essential part of any respiratory consultation, as poor disease control could indicate poor technique (GINA, 2020; UKIG, 2016). Single-use placebo inhalers and devices are available to check patients’ inhaler technique.
Spacer device
A spacer is a large holding chamber with a valve; it can be used with a pMDI to help patients who have problems co-ordinating activation of their inhaler and inhaling at the right time (Lister et al, 2020; Asthma UK, 2017).
A spacer:
- Slows the aerosol as it comes out of the inhaler, giving more time for it to reach the lungs;
- Reduces the ‘cold freon’ effect, which is a chilling sensation that hits the back of the throat following actuation of an inhaler and prevents the patient from continuing to inhale;
- Reduces the risk of side-effects of inhaled medicine, such as oral candidiasis.
Infection prevention
Inhalers and spacers are prescribed for individual patients and are for single patient use. New spacers should be washed before use with warm water and detergent, and then air dried without rinsing. Using a cloth or paper towel can result in static on the inside of the spacer, which makes the drug stick to the sides. The mouthpiece should be wiped to remove residual detergent before use (Asthma UK, 2017). Patients should repeat this cleaning process every month and after recovery from a cold or respiratory infection. If the patient has had Covid-19, a replacement spacer should be supplied.
Health professionals do not routinely require non-sterile gloves for administration of inhaled medicines. Nurses need to assess individual patients for the risk of exposure to blood and body fluids (Royal College of Nursing, 2018), and to be aware of local policies for use of personal protective equipment (PPE) when caring for patients with respiratory infections, such as Covid-19.
Environmental factors
The manufacture and disposal of pMDIs are a significant contributor to the NHS’s carbon footprint. The propellants (fluorinated gases) known as ‘greenhouse gases’, also contribute to climate change. Nurses may offer lower-carbon options if the device is suitable for the individual patient’s needs (Scullion, 2020). The National Institute for Health and Care Excellence (2019) asthma guideline has a decision aid, which includes consideration of the carbon footprint.
Procedure for teaching patients to use a pMDI
Equipment:
- Inhaler;
- Prescription chart;
- Spacer device if required.
If the inhaler is new or has not been used for a few weeks, the patient should be advised to test the device by removing the cap, shaking the inhaler and releasing a dose into the air. This should not be done every time the device is used (UKIG, 2016). Some inhalers have a dose counter, which shows the number of doses left in the inhaler device.
- Introduce yourself to the patient and assess their level of knowledge about their inhaler and their technique. If this is the first time they have used an inhaler, ensure they understand the rationale for each step in the procedure and use illustrated guides to support the key points.
- Gain the patient’s consent for administration of the inhaler.
- Wash and dry hands and apply PPE if required, and ask the patient to wash and dry their hands.
- Check and document the patient’s baseline observations of pulse (some inhalers can cause tachycardia), respirations and breath sounds before administration, so that effectiveness and any adverse effects can be measured after treatment.
- Help the patient to sit upright in a chair with feet flat on the floor or upright in bed to allow for full chest expansion.
- Check the prescription against the inhaler according to local guidelines for the administration of medicines, ensuring you follow the ‘five rights’ for medicines administration (Box 1).
- Check the patient’s identity following local guidelines for the administration of medicines. Check for any allergies.
- Hold the inhaler in an upright position; remove the cover and show the patient how to check for any foreign objects in the mouthpiece that could be inhaled.
- Ask the patient to shake the device for 2-3 seconds to mix the medication in the inhaler (Lister et al, 2020) (Fig 1a).
- Ask the patient to take a deep breath in, exhale completely and place the mouthpiece between their lips and teeth and ensure their lips are sealed tightly around the mouthpiece (Fig 1b).
- The patient should tip their head back slightly, which helps the medication reach the lungs. When using a pMDI, they should inhale slowly and deeply for 2-3 seconds while pressing down on the inhaler canister (Fig 1c).
- The patient should remove the inhaler from their mouth and hold their breath for 10 seconds and keep their lips pursed and then exhale (Fig 1d).
- Replace the cap on the inhaler.
- If the dose is to be repeated, ask the patient to wait 20-30 seconds between inhalations to allow the medicine to have an effect and reduce the risk of side-effects (Lister et al, 2020).
- If a different medication is required wait 2-3 minutes. Always administer bronchodilators before steroids as the bronchodilators help to open up the airway and allow greater disposition of the steroid in the lungs.
- The patient should rinse their mouth 2 minutes after taking inhaled steroids to reduce the risk of fungal infection, as steroids alter the normal flora of the oral cavity (Lister, 2020).
- Make the patient comfortable and clean the mouthpiece of the inhaler if necessary.
- Record the administration of the medicine according to local policy and its effectiveness.
- Wash and dry hands.
Box 1. The ‘five rights’ of medicine administration
- Right patient
- Right medicine
- Right route
- Right time
- Right dose

Spacers
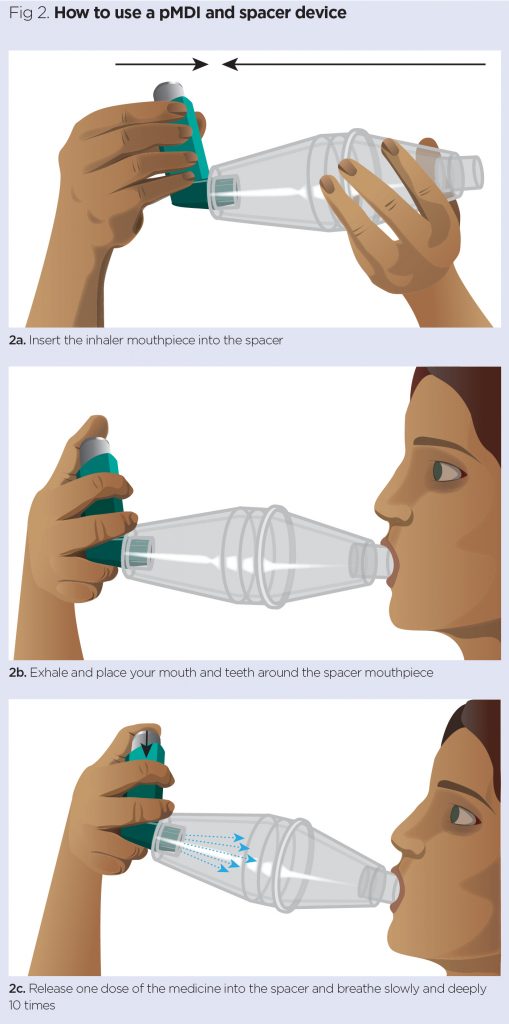
If a spacer is used follow stages 1-9 and rejoin at step 14.
- Attach the mouthpiece to a compatible spacer device (Fig 2a);
- Ask the patient to hold the inhaler and device between their index finger and thumb;
- The patient should exhale and place their mouth and teeth around the spacer mouthpiece (Fig 2b);
- Release one dose of the medicine into the spacer and ask the patient to breath slowly and deeply 1o times and then remove the mouthpiece (Fig 2c);
- Repeat if a second dose is required.
Asthma UK (2017) Spacer Devices. Asthma UK.
Global Initiative for Asthma (2020) Global Strategy for Asthma Management and Prevention. GINA.
Global Initiative for Chronic Obstructive Lung Disease (2020) Global Strategy for the Diagnosis, Management and Prevention of Chronic Obstructive Pulmonary Disease. GOLD.
Lister S et al (2020) The Royal Marsden Hospital Manual of Clinical Nursing Procedures. Wiley Blackwell.
Melani AS et al (2011) Inhaler mishandling remains common in real life and is associated with reduced disease control. Respiratory Medicine; 105: 930−938.
Murphy A (2016) How to help patients optimise their inhaler technique. The Pharmaceutical Journal (online). 27 July.
National Institute for Health and Care Excellence (2019) Patient Decision Aid for Asthma. NICE.
Royal College of Nursing (2018) Tools of the Trade: Guidance for Health Professionals on Glove Use and the Prevention of Contact Dermatitis. RCN.
Sanchis J et al (2016) Systematic review of errors in inhaler use: has patient technique improved over time? Chest; 150: 394–406.
Scullion J (2020) Sustainability 3: how nurses can reduce the environmental impact of inhalers. Nursing Times; 116: 9, 38-40.
Scullion J (2017) Standards and competencies to improve inhaler technique. Independent Nurse (online). 6 February.
Tena AF, Clarà PC (2012) Deposition of inhaled particles in the lungs. Archivos de Bronconeumología; 48; 7: 221-264.
UK Inhaler Group (2019) Inhaler Standards and Competency Document. UKIG.
