The medicines regulator has urged GPs to be alert to signs of ‘misuse’ of GLP-1 receptor agonists for weight loss, such as semaglutide.
Healthcare professionals should also ensure patients are aware of the side-effects of the diabetes and weight loss injections and report any adverse reactions through the Yellow Card reporting scheme, the Medicines and Healthcare products Regulatory Agency said.
The Government said the drugs were not a ‘quick fix’ and should only be used for approved indications, not for cosmetic reasons.
When used appropriately the benefits of the drugs outweigh the risks for patients, the MHRA said.
But that benefit-risk balance is only positive for patients within the approved indications for weight management or type 2 diabetes as described in the product information.
In addition, patients obtaining a private prescription should ensure this is dispensed from an authorised source, such as a registered pharmacy, to avoid the risk of obtaining a counterfeit medicine.
Some fake versions of the injectable have been found to contain insulin with patients needing urgent medical attention after use, the MHRA said.
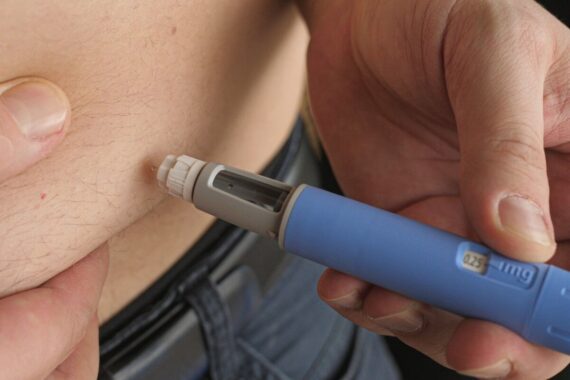
It is vital for patients to carefully read the instructions in the patient information leaflet and use the prescribed dose, the regulator added.
Potential side effects of these medicines can include gastrointestinal conditions, such as vomiting and diarrhoea, which will be mild for most people but can lead to more serious complications resulting in hospitalisation.
Healthcare professionals should also discuss the risk of serious, but less common side effects such as pancreatitis and gall bladder disorders, the MHRA said.
In addition, patients need to be made aware of the symptoms of hypoglycaemia that can occur in some non-diabetic patients using the drugs and what to do if it happens.
Patients prescribed GLP-1 receptor agonists should speak to a healthcare professional if they have any questions about potential side effects, the MHRA added.
MHRA chief safety officer, Dr Alison Cave, said: ‘All medicines carry a risk of potential side effects and GLP-1RAs are no exception.
‘We encourage healthcare professionals to ensure patients being treated with these medicines are aware of the common side effects and how to minimise risk.
‘The balance of benefits and risks outside the licensed indication has not been shown to be favourable. Please report cases of misuse especially if harm occurs.’
NHS England had asked NICE to consider plans for a slow phased roll out of tirzepatide for weight loss to avoid overwhelming GPs with demand.
Primary care weight loss services need to be put in place to provide the wrap around care and support that patients will need, the proposal said.
GPs have spoken about their concerns around shortages of the GLP-1RA drugs for patients with type 2 diabetes who really need them because of the global demand for them as aids to weight loss.
Health and social care secretary Wes Streeting said: ‘Weight-loss drugs have enormous potential. When taken alongside healthy diet and exercise, they can be game changers in tackling obesity and getting people back to good health.
‘But these are not cosmetic drugs that should be taken to help get a body beautiful picture for Instagram.
‘These are serious medicines and should only be used responsibly and under medical supervision. They’re not a quick fix to lose a few pounds and buying them online without appropriate assessment can put people’s health at risk.
‘Drugs approved for weight management should only be used by those tackling obesity, where diet and exercise has been tried first, and where patients are eligible.’








As an adjunct for getting people back to work?
The majority of prescribing is now done in the private sector, often not by GPs. Why does the MHRA only target GPs in particular. it should go out to all prescribers with an emphasis on training and safety. The people prescribing bear the responsibility for any problems in the future. Patients need to go back to them for problems as well.
Yea, Nelly. Policing inappropriate prescription and supply by non-doctors, and ‘direct-to-patient’ marketting of unlicensed medicinal products by unscrupulous commercial interests is not the job of GPs.
I wonder who’se job it is? Oh, the MHRA isn’t it?
Why have they allowed direct-to-patient marketting and supply without an assessment by a medical professional to start with?
A bit late to recruit busy GPs to try to rebuild the stable door after they torn it off it’s hinges.
“Please report cases of misuse”
No doubt there are plenty of doctors who will be only too happy to act as secret policeman on behalf of the State. Authoritarianism and left-wing politics are symbiotic and widespread, and not just in the BMA.