Precision medicine is rapidly having an impact on how drugs are developed, how patients are treated, and how health care delivery is channeling its resources to maximize patient benefits. In this post, we describe 5 key benefits of the movement and its potential to revolutionize the future research and medical landscape.
What Is Precision Medicine?
Precision medicine (PM) is the tailoring of medical treatment to the individual characteristics of each patient. PM tells us how a person’s unique genetic profile makes them susceptible to certain diseases, and is increasing our ability to predict which medical treatments will be safe and effective for certain patient populations.
This more effective approach toward prescribing means that, physicians can select a therapy or treatment protocol based on a patient’s molecular profile that minimizes adverse drug reactions (ADRs) and ensures a more successful treatment outcome. PM can also help contain costs compared with a “trial-and-error” approach to disease treatment, and has the potential to change the way we think about, identify and manage health problems.
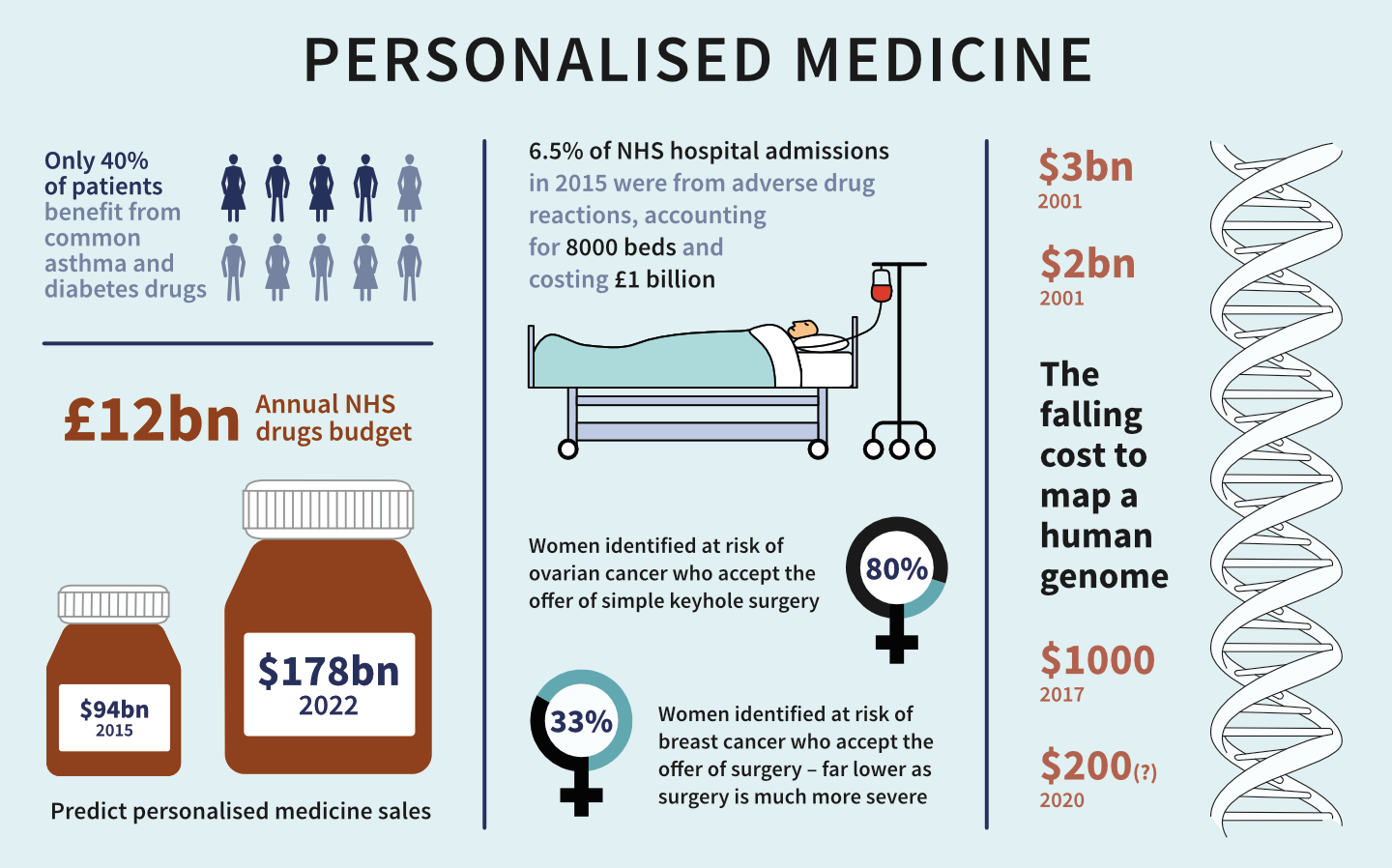
1. Shifts the medical emphasis from reaction to prevention
PM introduces the ability to use molecular markers that signal disease risk or presence before clinical signs and symptoms appear. It therefore offers the opportunity to focus on prevention and early intervention rather than on reaction at advanced stages of disease.
In many areas, early clinical interventions can be life-saving. For example, women with certain BRCA1 or BRCA2 gene variations have up to an 85% lifetime chance of developing breast cancer, and up to a 60% chance of developing ovarian cancer. The BRCA1 and BRCA2 genetic test can guide preventive measures, such as increased frequency of mammography, prophylactic surgery, and chemoprevention.
Currently, there are more than 15,000 tests for more than 2,800 genes. These tests can identify inherited susceptibility to conditions, ranging from hearing loss to sudden cardiac arrest. A subset of these tests have a predictive capability, meaning the ability to spot the potential disease before symptoms appear.
Although not every test is linked to a therapeutic option, a genetic diagnosis often permits targeted prevention strategies; it also can help eliminate the need for further costly and invasive diagnostic testing. A patient who learns he or she has inherited cardiomyopathy, for example, can benefit from suggested lifestyle changes and disease-monitoring options to avoid the risk of sudden death.

2. Reduces trial-and-error prescribing
Up to 50% of patients do not benefit from the first drug they are offered in treatment. Studies have linked these differences in response to the differences in genes that code for drug-metabolizing enzymes, drug transporters, or drug targets. The use of genetic and other forms of molecular screening therefore allows the physician to select an optimal therapy the first time, thus avoiding the frustrating and costly practice of trial-and error prescribing.
One of the most common applications of this practice has been for women with breast cancer. About 30% of breast cancer cases are characterized by overexpression of a cell surface protein called human epidermal growth factor receptor 2 (HER2). For women with this form of the disease, an antibody drug called Herceptin® (trastuzumab) can half the recurrence of a tumor when used in combination with chemotherapy.
Molecular diagnostic tests for HER2 are used to identify the patients who will benefit from receiving Herceptin® and other drugs that target HER2, such as Tykerb® (lapatinib). Two complex diagnostic tests, Oncotype DX® and MammaPrint,® for example, use genetic information to help physicians chart the best course of treatment for breast cancer patients.
Oncotype DX® can determine whether women with certain types of breast cancer are likely to benefit from chemotherapy. MammaPrint® can detect which early-stage breast cancer patients are at risk of distant recurrence following surgery. Both tests place patients into risk categories that inform physicians whether the cancer may be treated successfully with hormone therapy alone, avoiding the toxic effects of chemotherapy.

3. Helps avoid adverse drug reactions
The life sciences community strives to improve the safety and efficacy of its products, but much more work remains. Progress in developing and adopting diagnostics to identify which medicines work best for which patients, has been slow. In fact, between 2000 and 2011, the number of adverse events recorded by the FDA nearly tripled. According to several studies, about 5.3% of all hospital admissions are associated with ADRs.
Many ADRs result from variations in genes that code for drug-metabolizing enzymes, such as cytochrome P2C9 (CYp2C9). Administration of the drug warfarin, used to prevent blood clots, is complicated by genetic variations in this drug-metabolizing enzyme and an enzyme that activates vitamin K (VKORC1).
Warfarin dosing is typically adjusted for the individual patient through multiple rounds of trial-and-error, during which the patient may be at risk of excessive bleeding or further blood clots.The FDA now recommends genotyping for all patients before warfarin treatment, which allows for more precise dosing. Although the data are still evolving, early evidence suggests that this helps patients avoid serious and possibly fatal ADRs.

4. Increases patient adherence to treatment
Patient non-compliance with treatment leads to adverse health effects and increased overall health care costs. Yet patients may be more likely to comply with their treatments when personalized therapies prove more effective or present fewer side effects. The greatest impact could be for the treatment of chronic diseases, such as asthma and diabetes, in which non-compliance commonly exacerbates the condition.
Knowledge of a genetic predisposition for a condition also provides patients with a powerful incentive to make lifestyle changes and manage their condition. In one study, patients with a genetic diagnosis showed more than 86% adherence to their treatment program after two years, compared to 38% prior to testing.
5. Reveals additional or alternative uses for drug candidates
A medicine that may show weaker efficacy in a more generalized patient population may show greater benefits when its use is limited to genetically defined patient populations. For example, the lung cancer drug Iressa® (gefitinib) did not demonstrate a survival advantage in a general population of patients in clinical trials, and was withdrawn from the market after initially being granted accelerated approval.
However, the sponsoring company has been using pharmacogenetics to demonstrate benefit in about 10% of patients who test positive for epidermal growth factor mutations, and it has won approval as a first-line treatment for that patient population in the UK.

Conclusion
PM offers significant short- and long-term benefits, especially for chronic and complex diseases. Payment and reimbursement policies should not discourage interventions that may raise short-term costs but improve clinical and cost value over time.
Policies that recognize the principles of PM will allow physicians to individualize treatment plans for patients through the early diagnosis of disease, target treatments to optimize clinical outcomes, and prevent unnecessary hospitalizations and care, thus reducing long-term costs.
Innovators are responsible for developing the collective evidence to justify the contention that PM can improve outcomes while controlling costs. Except in the case of some individual products, to date they have not proven that contention. When they do, our argument will be more compelling.
Learn how REPROCELL are helping develop PM in Scotland in the report: Precision Medicine Innovation in Scotland: Accelerating Productivity Growth for Scotland and the UK.







