Description for Cantil
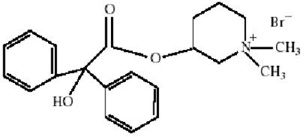
CANTIL tablets for oral administration contain 25 mg mepenzolate bromide USP. The anticholinergic agent mepenzolate bromide USP chemically is 3-[(hydroxydiphenylacetyl)oxy]-1,1- dimethylpiperidinium bromide and has the following structure:
 |
Mepenzolate bromide occurs as a white or light cream-colored powder, which is freely soluble in methanol, slightly soluble in water and chloroform, and practically insoluble in ether.
Each yellow tablet contains 25 mg mepenzolate bromide USP. This tablet also contains inactive ingredients: confectioners' sugar, corn starch, corn syrup solids, FD&C Yellow No. 5 (tartrazine), (see PRECAUTIONS, General), lactose, magnesium stearate and microcrystalline cellulose.
Uses for Cantil
CANTIL is indicated for use as adjunctive therapy in the treatment of peptic ulcer. It has not been shown to be effective in contributing to the healing of peptic ulcer, decreasing the rate of recurrence, or preventing complications.
Dosage for Cantil
The usual adult dose is 1 or 2 tablets (25 or 50 mg) 4 times a day preferably with meals and at bedtime. Begin with the lower dosage when possible and adjust subsequently according to the patient's response.
Safety and efficacy in pediatric patients have not been established.
In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal or cardiac function, and of concomitant disease or other drug therapy. (See PRECAUTIONS, Geriatric Use.)
HOW SUPPLIED
25 mg mepenzolate bromide, compressed yellow tablets debossed MERRELL 37.
NDC 0068-0037-01: bottles of 100
Keep tightly closed. Store at room temperature, preferably below 86°F. Protect from excessive heat. Dispense in tight containers with child-resistant closure.
Manufactured for: sanofi-aventis U.S. LLC, Bridgewater, NJ 08807. Revised: Feb 2006
Side Effects for Cantil
Precise frequency data from controlled clinical studies with CANTIL are not available.
Gastrointestinal System: vomiting, nausea, constipation, loss of taste, bloated feeling, dry mouth
Central Nervous System: mental confusion, dizziness, weakness, drowsiness, headache, nervousness
Ophthalmologic: increased ocular tension, cycloplegia, blurred vision, dilation of the pupil
Dermatologic-Hypersensitivity: anaphylaxis, urticaria
Cardiovascular: tachycardia, palpitations
Genitourinary: urinary retention, urinary hesitancy
Miscellaneous : decreased sweating, drowsiness, insomnia, impotence, suppression of lactation
Drug Abuse And Dependence
Tolerance, abuse, or dependence has not been reported with CANTIL.
Drug Interactions for Cantil
The following agents may increase certain actions or side effects of anticholinergic drugs: amantadine, antiarrhythmic agents of class I (e.g., quinidine), antihistamines, antipsychotic agents (e.g., phenothiazines), benzodiazepines, MAO inhibitors, narcotic analgesics (e.g., meperidine), nitrates and nitrites, sympathomimetic agents, tricyclic antidepressants, and other drugs having anticholinergic activity.
Anticholinergics antagonize the effects of antiglaucoma agents. Anticholinergic drugs in the presence of increased intraocular pressure may be hazardous when taken concurrently with agents such as corticosteroids. (See CONTRAINDICATIONS.)
Anticholinergic agents may affect gastrointestinal absorption of various drugs, such as slowly dissolving dosage forms of digoxin; increased serum digoxin concentrations may result. Anticholinergic drugs may antagonize the effects of drugs that alter gastrointestinal motility, such as metoclopramide. Because antacids may interfere with the absorption of anticholinergic agents, simultaneous use of these drugs should be avoided.
The inhibiting effects of anticholinergic drugs on gastric hydrochloric acid secretion are antagonized by agents used to treat achlorhydria and those used to test gastric secretion.
Warnings for Cantil
In the presence of high environmental temperature, heat prostration (fever and heat stroke due to decreased sweating) can occur with use of CANTIL.
Diarrhea may be an early symptom of incomplete intestinal obstruction especially in patients with ileostomy or colostomy. In this instance, treatment with this drug would be inappropriate and possibly harmful.
CANTIL may produce drowsiness or blurred vision. The patient should be cautioned regarding activities requiring mental alertness such as operating a motor vehicle or other machinery or performing hazardous work while taking this drug.
With overdosage, a curare-like action may occur i.e., neuromuscular blockage leading to muscular weakness and possible paralysis.
It should be noted that the use of anticholinergic drugs in the treatment of gastric ulcer may produce a delay in gastric emptying time and may complicate such therapy (antral stasis).
Psychosis has been reported in sensitive individuals given anticholinergic drugs. CNS signs and symptoms include confusion, disorientation, short-term memory loss, hallucinations, dysarthria, ataxia, coma, euphoria, decreased anxiety, fatigue, insomnia, agitation and mannerisms and inappropriate affect.
These CNS signs and symptoms usually resolve within 12 to 24 hours after discontinuation of the medication.
Precautions for Cantil
General
Use CANTIL with caution in the elderly (see PRECAUTIONS, Geriatric Use) and in all patients with:
- Autonomic neuropathy
- Hepatic or renal disease
- Ulcerative colitis. Large doses may suppress intestinal motility to the point of producing a paralytic ileus and for this reason precipitate or aggravate “toxic megacolon,” a serious complication of the disease.
- Hiatal hernia associated with reflux esophagitis, since anticholinergic drugs may aggravate this condition.
- Coronary heart disease
- Congestive heart failure
- Cardiac arrhythmias
- Tachycardia
- Hypertension
- Prostatic hypertrophy
- Hyperthyroidism
Investigate any tachycardia before giving anticholinergic (atropine-like) drugs since they may increase the heart rate.
This product contains FD&C Yellow No. 5 (tartrazine), which may cause allergic-type reactions (including bronchial asthma) in certain susceptible individuals. Although the overall incidence of FD&C Yellow No. 5 (tartrazine) sensitivity in the general population is low, it is frequently seen in patients who also have aspirin sensitivity.
Carcinogenesis, Mutagenesis, Impairment Of Fertility
No data are available on long-term potential for carcinogenicity, mutagenicity, or impairment of fertility in animals or humans.
Pregnancy
Teratogenic Effects
Pregnancy Category B. Reproduction studies have been performed in rats and rabbits at doses up to 30 times the human dose (based on 50 kg weight) and have shown no evidence of impaired fertility or harm to the animal fetus. There are, however, no adequate and well-controlled studies with CANTIL in pregnant women. Because animal reproduction studies are not always predictive of human response, CANTIL should be used during pregnancy only if clearly needed.
Nonteratogenic Effects
No data are available on nonteratogenic effects in the fetus or newborn infant.
Nursing Mothers
It is not known whether CANTIL is secreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when CANTIL is administered to a nursing woman.
Pediatric Use
Safety and effectiveness in pediatric patients have not been established. Studies in newborn animals (rats) show that younger animals are more sensitive to the toxic effects of CANTIL than are older animals.
Geriatric Use
Clinical studies of CANTIL did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range (see DOSAGE AND ADMINISTRATION), reflecting the greater frequency of decreased hepatic, renal or cardiac function (see PRECAUTIONS, General), and of concomitant disease or other drug therapy. (See DRUG INTERACTIONS.)
CANTIL is contraindicated in intestinal atony of the elderly. (See CONTRAINDICATIONS.) CANTIL should be used with caution in the elderly. (See PRECAUTIONS, General.)
Overdose Information for Cantil
Signs And Symptoms
The signs and symptoms of overdosage are headache; nausea; vomiting; blurred vision; dilated pupils; hot, dry skin; dizziness; dryness of the mouth; difficulty in swallowing; and CNS stimulation. A curarelike action may occur (i.e., neuromuscular blockade leading to muscular weakness and possible paralysis).
Oral LD50
The oral LD50 is greater than 750 mg/kg in mice and greater than 1000 mg/kg in rats.
Maximum Human Dose Recorded
The maximum human dose recorded is 375 to 500 mg in a 4-year-old child (no adverse effects reported) and 500 to 750 mg in a 30-year-old adult (resulted in death).
Dialysis
It is not known if the drug is dialyzable.
Treatment
Treatment should consist of gastric lavage, emetics, and activated charcoal. Sedatives (e.g., short-acting barbiturates, benzodiazepines) may be used for management of overt signs of excitement. If indicated, an appropriate parenteral cholinergic agent may be used as an antidote.
Contraindications for Cantil
- Glaucoma
- Obstructive uropathy (for example, bladder neck obstruction due to prostatic hypertrophy)
- Obstructive disease of the gastrointestinal tract (for example, pyloroduodenal stenosis, achalasia)
- Paralytic ileus
- Intestinal atony of the elderly or debilitated patient (See PRECAUTIONS, Geriatric Use)
- Unstable cardiovascular status in acute gastrointestinal hemorrhage
- Toxic megacolon complicating ulcerative colitis
- Myasthenia gravis
- Allergic or idiosyncratic reactions to CANTIL or related compounds
Clinical Pharmacology for Cantil
CANTIL diminishes gastric acid and pepsin secretion. CANTIL also suppresses spontaneous contractions of the colon. Pharmacologically, it is a post-ganglionic parasympathetic inhibitor. Radiotracer studies in which CANTIL- 14C was used in animals and humans indicate the absorption following oral administration, as with other quaternary ammonium compounds, is low. Between 3 and 22% of an orally administered dose is excreted in the urine over a 5-day period, with the majority of the radioactivity appearing on Day 1. The remainder appears in the next 5 days in the feces and presumably has not been absorbed.
From 

Report Problems to the Food and Drug Administration
You are encouraged to report negative side effects of prescription drugs to the FDA. Visit the FDA MedWatch website or call 1-800-FDA-1088.