Description for Jelmyto
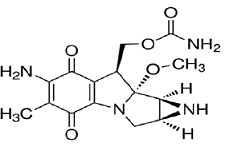
Mitomycin (also known as mitomycin-C) is an alkylating drug isolated from the broth of Streptomyces caespitosus. Mitomycin is a blue-violet crystalline powder with a molecular formula of C15H18N4O5, and a molecular weight of 334.33. Its chemical name is 7-amino-9α-methoxymitosane and it has the following structural formula:
 |
Mitomycin is heat stable, has a high melting point, and is freely soluble in organic solvents.
Jelmyto is supplied in a single-dose carton containing two vials of sterile lyophilized mitomycin for pyelocalyceal solution, 40 mg each, and one vial of 20 mL of sterile hydrogel, to be used as a vehicle for reconstitution.
Mitomycin for pyelocalyceal solution is a sterile, lyophilized, grey to greyish-purple, cake or powder that contains mitomycin 40 mg and mannitol 80 mg in each vial.
Sterile hydrogel is a sterile, clear, colorless, gel with or without bubbles at room temperature or clear, colorless liquid at 2°C to 8°C (36°F to 46°F), which contains 0.04 g hydroxypropyl methylcellulose, 5.67 g poloxamer, 0.21 g polyethylene glycol, and water for injection in each vial.
Once reconstituted, Jelmyto is a clear, purple, viscous liquid at 2°C to 8°C (36°F to 46°F) or semisolid gel at room temperature with a concentration of 4 mg per mL of mitomycin, which may contain a few visible particles and have a pH between 6.0 and 8.0.
From 

Report Problems to the Food and Drug Administration
You are encouraged to report negative side effects of prescription drugs to the FDA. Visit the FDA MedWatch website or call 1-800-FDA-1088.