WARNING
ESAs INCREASE THE RISK OF DEATH, MYOCARDIAL INFARCTION, STROKE, VENOUS THROMBOEMBOLISM, THROMBOSIS OF VASCULAR ACCESS AND TUMOR PROGRESSION OR RECURRENCE.
Chronic Kidney Disease:
In controlled trials, patients experienced greater risks for death, serious adverse cardiovascular reactions, and stroke when administered erythropoiesis-stimulating agents (ESAs) to target a hemoglobin level of greater than 11 g/dL.
No trial has identified a hemoglobin target level, ESA dose, or dosing strategy that does not increase these risks [see WARNINGS AND PRECAUTIONS].
Use the lowest OMONTYS dose sufficient to reduce the need for red blood cell (RBC) transfusions [see WARNINGS AND PRECAUTIONS].
Description for Omontys
Peginesatide is an ESA that is a synthetic, pegylated dimeric peptide comprised of two identical, 21-amino acid chains covalently bonded to a linker derived from iminodiacetic acid and β-alanine. Peginesatide is manufactured as an acetate salt. The dimeric peptide (approximate MW 4,900 daltons) is covalently linked to a single lysine-branched bis-(methoxypoly(ethylene glycol)) (PEG) chain (approximate MW 40,000 daltons). The structure is provided in Figure 1; peginesatide has no amino acid sequence homology to erythropoietin. The empirical formula is C2031H3950N62O958S6 (free base). The total molecular weight is approximately 45,000 daltons.
Figure 1: Structure of peginesatide acetate
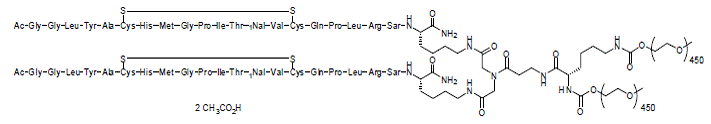 |
OMONTYS (peginesatide) Injection is formulated as a sterile, colorless to slightly yellow preservative-free solution and a sterile, colorless to slightly yellow preserved solution for intravenous or subcutaneous administration.
Single use, preservative-free injectable solutions of OMONTYS in vials and pre-filled syringes are supplied in multiple strengths. Each 0.5 mL vial or pre-filled syringe contains peginesatide, 23.5 mg sorbitol, 1.5 mg sodium phosphate monobasic (dihydrate), 0.06 mg sodium phosphate dibasic, 0.02 mg polysorbate 20, and water for injection, USP. Sodium hydroxide added to adjust pH. The pH is 6.0 ± 0.3.
Multiple use, preserved injectable solutions of OMONTYS in vials are supplied in two fill volumes: 1 mL and 2 mL. Each mL contains 10 mg peginesatide, 47 mg sorbitol, 5 mg phenol, 1.5 mg L-methionine, 0.6 mg glacial acetic acid, and water for injection, USP. Sodium hydroxide added to adjust pH. The pH is 5.4 ± 0.5.
Uses for Omontys
Anemia Due to Chronic Kidney Disease
OMONTYS® is indicated for the treatment of anemia due to chronic kidney disease (CKD) in adult patients on dialysis.
Limitations of Use
OMONTYS is not indicated and is not recommended for use:
- In patients with CKD not on dialysis because of safety concerns in this population [see WARNINGS AND PRECAUTIONS].
- In patients receiving treatment for cancer and whose anemia is not due to CKD, because ESAs have shown harm in some settings and the benefit-risk factors for OMONTYS in this setting have not been evaluated [see WARNINGS AND PRECAUTIONS].
- As a substitute for RBC transfusions in patients who require immediate correction of anemia.
- OMONTYS has not been shown to improve symptoms, physical functioning or health-related quality of life.
Dosage for Omontys
Evaluation of Iron Stores and Nutritional Factors
Evaluate the iron status in all patients before and during treatment and maintain iron repletion. Correct or exclude other causes of anemia (e.g., vitamin deficiency, metabolic or chronic inflammatory conditions, bleeding, etc.) before initiating OMONTYS [see WARNINGS AND PRECAUTIONS].
Patients with Chronic Kidney Disease
Individualize dosing and use the lowest dose of OMONTYS sufficient to reduce the need for RBC transfusions [see WARNINGS AND PRECAUTIONS]. In controlled trials, patients experienced greater risks for death, serious adverse cardiovascular reactions, and stroke when administered erythropoiesis-stimulating agents (ESAs) to target a hemoglobin level of greater than 11 g/dL. No trial has identified a hemoglobin target level, ESA dose, or dosing strategy that does not increase these risks. Physicians and patients should weigh the possible benefits of decreasing transfusions against the increased risks of death and other serious cardiovascular adverse events [see BOXED WARNING and Clinical Studies].
Initiation of Treatment and Starting Dose
Initiate OMONTYS treatment when the hemoglobin level is less than 10 g/dL.
The recommended starting dose for the treatment of anemia in patients who are not currently treated with an ESA is 0.04 mg/kg body weight administered as a single intravenous or subcutaneous injection once monthly.
Conversion from Epoetin Alfa or Darbepoetin Alfa to OMONTYS in Patients with CKD on Dialysis
OMONTYS is administered once monthly, either subcutaneously or intravenously.
In patients previously receiving epoetin alfa or darbepoetin alfa, estimate the starting monthly dose of OMONTYS for patients on the basis of the weekly dose of epoetin alfa or darbepoetin alfa at the time of substitution (see Table 1). Maintain the route of administration (intravenous or subcutaneous injection).
- For patients previously receiving epoetin alfa, the first dose of OMONTYS should be administered one week after the last epoetin alfa dose was administered.
- For patients previously receiving darbepoetin alfa, the first dose of OMONTYS should be administered at the next scheduled dose in place of darbepoetin alfa.
Table 1 : Estimated OMONTYS Starting Doses for
Patients Based on Previous Weekly ESA Dose
| Previous Total Weekly Epoetin Alfa Dose (U/week) | Previous Weekly Darbepoetin Alfa Dose (mcg/week) | OMONTYS Dose Once Monthly (mg/month) |
| Less than 2,500 | Less than 12 | 2 |
| 2,500 to less than 4,300 | 12 to less than 18 | 3 |
| 4,300 to less than 6,500 | 18 to less than 25 | 4 |
| 6,500 to less than 8,900 | 25 to less than 35 | 5 |
| 8,900 to less than 13,000 | 35 to less than 45 | 6 |
| 13,000 to less than 19,000 | 45 to less than 60 | 8 |
| 19,000 to less than 33,000 | 60 to less than 95 | 10 |
| 33,000 to less than 68,000 | 95 to less than 175 | 15 |
| greater than or equal to 68,000 | greater than or equal to 175 | 20 |
General Guidance including Dose Adjustments
Monitor hemoglobin levels at least every 2 weeks until stable, then monitor at least monthly. When adjusting therapy, consider hemoglobin rate of rise, rate of decline, ESA responsiveness and hemoglobin variability. A single hemoglobin excursion may not require a dosing change.
- Do not increase the dose more frequently than once every 4 weeks.
- If the hemoglobin rises rapidly (e.g., more than 1 g/dL in the 2 weeks prior to the dose or more than 2 g/dL in 4 weeks), reduce the dose of OMONTYS by 25% or more as needed to reduce rapid responses.
- If the hemoglobin level approaches or exceeds 11 g/dL, reduce or interrupt the dose of OMONTYS. After a dose has been withheld and once the hemoglobin begins to decrease, OMONTYS may be restarted at a dose approximately 25% below the previously administered dose.
- For patients who do not respond adequately, if the hemoglobin has not increased by more than 1 g/dL after 4 weeks of therapy, increase the dose by 25%.
- For patients who do not respond adequately over a 12-week escalation period, increasing the OMONTYS dose further is unlikely to improve response and may increase risks. Use the lowest dose that will maintain a hemoglobin level sufficient to reduce the need for RBC transfusions. Evaluate other causes of anemia. Discontinue OMONTYS if responsiveness does not improve [see WARNINGS AND PRECAUTIONS].
- If a dose of OMONTYS is missed, administer the missed dose as soon as possible and restart OMONTYS at the prescribed once monthly dosing frequency.
When treating dialysis patients who have chronic kidney disease and cancer, physicians should refer to WARNINGS AND PRECAUTIONS.
Refer patients who self-administer OMONTYS to the Instructions for Use [see PATIENT INFORMATION].
Preparation and Administration of OMONTYS
OMONTYS is packaged as single use vials, single use pre-filled syringes, and multiple use vials. OMONTYS packaged in single use vials and single use pre-filled syringes contains no preservatives. OMONTYS is administered either subcutaneously or intravenously.
- Use the single use vial or single use pre-filled syringe only one time. Discard unused portion of OMONTYS in single use vials.
- Store unused portions of OMONTYS in multiple use vials at 36 °F to 46 °F (2 °C to 8 °C). Discard 28 days after first use.
- Protect OMONTYS from light. Store OMONTYS vials or pre-filled syringes in their cartons until time of use.
- Do not use if tamper-evident seal on carton is broken or missing.
- Do not dilute OMONTYS and do not administer in conjunction with other drug solutions.
- OMONTYS should be inspected visually for particulate matter and coloration prior to administration. Do not use any vials or pre-filled syringes of OMONTYS exhibiting particulate matter or a coloration other than colorless to slightly yellow.
HOW SUPPLIED
Dosage Forms And Strengths
| Dosage Form | Strengths |
| Single use vials (preservative-free) | 2 mg/0.5 mL, 3 mg/0.5 mL, 4 mg/0.5 mL, 5 mg/0.5 mL, and 6 mg/0.5 mL |
| Single use pre-filled syringes (preservative-free) | 1 mg/0.5 mL, 2 mg/0.5 mL, 3 mg/0.5 mL, 4 mg/0.5 mL, 5 mg/0.5 mL, and 6 mg/0.5 mL |
| Multiple use vials (with preservative) | 10 mg/mL and 20 mg/2 mL |
OMONTYS is available in single use vials, single use pre-filled syringes, and multiple use vials. The vial caps and plungers of the pre-filled syringes are designated with unique colors for each dosage strength. The single use pre-filled syringes are supplied with a 27 gauge, ½ inch needle. To reduce the risk of accidental needle sticks after application, each single use pre-filled syringe is equipped with a safety device that activates and covers the needle once the dose has been given.
OMONTYS is available in the following pack sizes:
| Single use vials | Single use pre-filled syringes with a 27 gauge, ½ inch needle with an UltraSafe Passive® Needle Guard | Multiple use vials |
| 1 Vial/Carton | 1 Syringe/Carton | 1 Vial/Carton |
| 2 mg/0.5 mL (NDC 64764-602-05) |
1 mg/0.5 mL (NDC 64764-601-99) |
10 mg/mL (NDC 64764-610-10) |
| 3 mg/0.5 mL (NDC 64764-603-05) |
2 mg/0.5 mL (NDC 64764-602-99) |
20 mg/2 mL (NDC 64764-620-20) |
| 4 mg/0.5 mL (NDC 64764-604-05) |
3 mg/0.5 mL (NDC 64764-603-99) |
|
| 5 mg/0.5 mL (NDC 64764-605-05) |
4 mg/0.5 mL (NDC 64764-604-99) |
|
| 6 mg/0.5 mL (NDC 64764-606-05) |
5 mg/0.5 mL (NDC 64764-605-99) |
|
| 6 mg/0.5 mL (NDC 64764-606-99) |
Stability and Storage
The recommended storage temperature is refrigerated at 2 °C to 8 °C (36 °F to 46 °F). Protect from light. Retain in carton until time of use.
Storage of single use vials, single use pre-filled syringes or multiple use vials over the recommended temperature (2 °C to 8 °C), when necessary, is permissible only for temperatures up to 25 °C (77 °F) and for no more than 30 days.
After first use, the multiple use vials should be stored at 2 °C to 8 °C, and then discarded after 28 days.
Marketed by: Affymax, Inc. Palo Alto, CA 94304. Distributed and Marketed by: Takeda Pharmaceuticals America, Inc. Deerfield, IL 60015. Revised: December 2012
Side Effects for Omontys
The following serious adverse reactions are discussed in greater detail in other sections of the labeling:
- Increased Mortality, Myocardial Infarction, Stroke, and Thromboembolism [see WARNINGS AND PRECAUTIONS]
- Hypertension [see WARNINGS AND PRECAUTIONS]
- Serious allergic reactions [see WARNINGS AND PRECAUTIONS]
Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical studies of OMONTYS cannot be directly compared to rates in the clinical trials of other drugs and may not reflect the rates observed in practice.
Patients with Chronic Kidney Disease
Adverse reactions were determined based on pooled data from two active controlled studies of 1066 dialysis patients treated with OMONTYS and 542 treated with epoetin, including 938 exposed for at least 6 months and 825 exposed for greater than one year to OMONTYS. The population for OMONTYS was 20 to 93 years of age, 58.5% male, and the percentages of Caucasian, Black (including African Americans), and Asian patients were 57.9%, 37.4%, and 3.1%, respectively. The median weight adjusted dose of OMONTYS was 0.07 mg/kg and 113 U/week/kg of epoetin.
Table 3 summarizes the most frequent adverse reactions ( ≥ 10%) in dialysis patients treated with OMONTYS.
Table 3 : Adverse Reactions Occurring in ≥ 10%
of Dialysis Patients treated with OMONTYS
| Adverse Reactions | Dialysis Patients Treated with OMONTYS (N = 1066) |
Dialysis Patients Treated with Epoetin (N = 542) |
| Gastrointestinal Disorders | ||
| Diarrhea | 18.4% | 15.9% |
| Nausea | 17.4% | 19.6% |
| Vomiting | 15.3% | 13.3% |
| Respiratory, Thoracic and Mediastinal Disorders | ||
| Dyspnea | 18.4% | 19.4% |
| Cough | 15.9% | 16.6% |
| Injury, Poisoning and Procedural Complications | ||
| Arteriovenous Fistula Site Complication | 16.1% | 16.6% |
| Procedural Hypotension | 10.9% | 12.5% |
| Nervous System Disorders | ||
| Headache | 15.40% | 15.90% |
| Musculoskeletal and Connective Tissue Disorders | ||
| Muscle Spasms | 15.3% | 17.2% |
| Pain in Extremity | 10.9% | 12.7% |
| Back Pain | 10.9% | 11.3% |
| Arthralgia | 10.7% | 9.8% |
| Vascular Disorders | ||
| Hypotension | 14.2% | 14.6% |
| Hypertension | 13.2% | 11.4% |
| General Disorders and Administration Site Conditions | ||
| Pyrexia | 12.20% | 14.00% |
| Metabolism and Nutrition Disorders | ||
| Hyperkalemia | 11.40% | 11.80% |
| Infections and Infestations | ||
| Upper Respiratory Tract Infection | 11.00% | 12.40% |
Seizures have occurred in patients participating in OMONTYS clinical studies. During the first several months following initiation of OMONTYS, blood pressure and the presence of premonitory neurologic symptoms should be monitored closely.
Advise patients to contact their healthcare practitioner for new-onset seizures, premonitory symptoms, or change in seizure frequency.
Allergic and infusion-related reactions have been reported in patients treated with OMONTYS.
Postmarketing Experience
Because postmarketing reporting of adverse reactions is voluntary and from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Serious allergic reactions have been reported during postmarketing use of OMONTYS [see WARNINGS AND PRECAUTIONS].
Immunogenicity
Of the 2357 patients tested during clinical trials, 29 (1.2%) had detectable levels of peginesatide-specific binding antibodies. There was a higher incidence of peginesatide-specific binding antibodies in patients dosed subcutaneously (1.9%) as compared to those dosed intravenously (0.7%). Peginesatide neutralizing antibodies were detected in vitro using a cell-based functional assay in 21 of these patients (0.9%). In approximately half of all antibody-positive patients, the presence of antibodies was associated with declining hemoglobin levels, the requirement for increased doses of OMONTYS to maintain hemoglobin levels, and/or transfusion for anemia of CKD. No cases of pure red cell aplasia (PRCA) developed in patients receiving OMONTYS during clinical trials.
Drug Interactions for Omontys
No formal drug/drug interaction studies have been performed. Peginesatide does not bind to serum albumin or lipoproteins as demonstrated in in vitro protein binding studies in rat, monkey and human sera. In vitro studies conducted with human hepatocytes or microsomes have shown no potential for peginesatide to induce or inhibit CYP450 enzymes.
Warnings for Omontys
Included as part of the PRECAUTIONS section.
Precautions for Omontys
Increased Mortality, Myocardial Infarction, Stroke, and Thromboembolism
- In controlled clinical trials of other ESAs in patients with CKD comparing higher hemoglobin targets (13 - 14 g/dL) to lower targets (9 - 11.3 g/dL) (see Table 2), increased risk of death, myocardial infarction, stroke, congestive heart failure, thrombosis of hemodialysis vascular access, and other thromboembolic events was observed in the higher target groups.
- Using ESAs to target a hemoglobin level of greater than 11 g/dL increases the risk of serious adverse cardiovascular reactions and has not been shown to provide additional benefit. Use caution in patients with coexistent cardiovascular disease and stroke [see DOSAGE AND ADMINISTRATION]. Patients with CKD and an insufficient hemoglobin response to ESA therapy may be at even greater risk for cardiovascular reactions and mortality than other patients. A rate of hemoglobin rise of greater than 1 g/dL over 2 weeks may contribute to these risks.
- In controlled clinical trials of ESAs in patients with cancer, increased risk for death and serious adverse cardiovascular reactions was observed. These adverse reactions included myocardial infarction and stroke.
- In controlled clinical trials, ESAs increased the risk of death in patients undergoing coronary artery bypass graft surgery (CABG) and deep venous thrombosis (DVT) was observed in patients undergoing orthopedic procedures.
The design and overall results of 3 large trials comparing higher and lower hemoglobin targets are shown in Table 2 (Normal Hematocrit Study (NHS), Correction of Hemoglobin Outcomes in Renal Insufficiency (CHOIR) and Trial to Reduce Cardiovascular Events with Aranesp® Therapy (TREAT)).
Table 2 : Adverse Cardiovascular Outcomes in
Randomized Controlled Trials Comparing Higher and Lower Hemoglobin Targets in
Patients with CKD
| NHS (N = 1265) |
CHOIR (N = 1432) |
TREAT (N = 4038) |
|
| Time Period of Trial | 1993 to 1996 | 2003 to 2006 | 2004 to 2009 |
| Population | Patients with CKD on hemodialysis with coexisting CHF or CAD, hematocrit 30 ± 3% on epoetin alfa | Patients with CKD not on dialysis with hemoglobin < 11 g/dL not previously administered epoetin alfa | Patients with CKD not on dialysis with type II diabetes, hemoglobin ? 11 g/dL |
| Hemoglobin Target; Higher vs. Lower (g/dL) | 14.0 vs. 10.0 | 13.5 vs. 11.3 | 13.0 vs. ? 9.0 |
| Median (Q1, Q3) Achieved Hemoglobin level (g/dL) | 12.6 (11.6, 13.3) vs. 10.3 (10.0, 10.7) | 13.0 (12.2, 13.4) vs. 11.4 (11.1, 11.6) | 12.5 (12.0, 12.8) vs. 10.6 (9.9, 11.3) |
| Primary Endpoint | All-cause mortality or non-fatal MI | All-cause mortality, MI, hospitalization for CHF, or stroke | All-cause mortality, MI, myocardial ischemia, heart failure, and stroke |
| Hazard Ratio or Relative Risk (95% CI) | 1.28 (1.06 – 1.56) | 1.34 (1.03 – 1.74) | 1.05 (0.94 – 1.17) |
| Adverse Outcome for Higher Target Group | All-cause mortality | All-cause mortality | Stroke |
| Hazard Ratio or Relative Risk (95% CI) | 1.27 (1.04 – 1.54) | 1.48 (0.97 – 2.27) | 1.92 (1.38 – 2.68) |
Patients with Chronic Kidney Disease Not on Dialysis
OMONTYS is not indicated and is not recommended for the treatment of anemia in patients with CKD who are not on dialysis.
A higher percentage of patients (22%) who received OMONTYS experienced a composite cardiovascular safety endpoint event compared to 17% who received darbepoetin alfa in two randomized, active-controlled, open-label, multi-center trials of 983 patients with anemia due to CKD who were not on dialysis. The trials had a pre-specified, prospective analysis of a composite safety endpoint consisting of death, myocardial infarction, stroke, or serious adverse events of congestive heart failure, unstable angina or arrhythmia (hazard ratio 1.32, 95% CI: 0.97, 1.81).
Increased Mortality and/or Increased Risk of Tumor Progression or Recurrence in Patients with Cancer receiving ESAs
OMONTYS is not indicated and is not recommended for reduction of RBC transfusions in patients receiving treatment for cancer and whose anemia is not due to CKD because ESAs have shown harm in some settings and the benefit-risk factors for OMONTYS in this setting have not been evaluated.
The safety and efficacy of OMONTYS have not been established for use in patients with anemia due to cancer chemotherapy. Results from clinical trials of ESAs in patients with anemia due to cancer therapy showed decreased locoregional control, progression-free survival and/or decreased overall survival. The findings were observed in clinical trials of other ESAs administered to patients with: breast cancer receiving chemotherapy, advanced head and neck cancer receiving radiation therapy, lymphoid malignancy, cervical cancer, non-small cell lung cancer, and with various malignancies who were not receiving chemotherapy or radiotherapy.
Hypertension
OMONTYS is contraindicated in patients with uncontrolled hypertension.
Appropriately control hypertension prior to initiation of and during treatment with OMONTYS. Reduce or withhold OMONTYS if blood pressure becomes difficult to control. Advise patients of the importance of compliance with antihypertensive therapy and dietary restrictions [see PATIENT INFORMATION].
Serious Allergic Reactions
Serious allergic reactions, including anaphylactic reactions, hypotension, bronchospasm, angioedema and generalized pruritus, may occur in patients treated with OMONTYS. Immediately and permanently discontinue OMONTYS and administer appropriate therapy if a serious allergic reaction occurs.
Lack or Loss of Response to OMONTYS
For lack or loss of hemoglobin response to OMONTYS, initiate a search for causative factors (e.g., iron deficiency, infection, inflammation, bleeding). If typical causes of lack or loss of hemoglobin response are excluded, evaluate the patient for the presence of antibodies to peginesatide. In the absence of antibodies to peginesatide, follow dosing recommendations for management of patients with an insufficient hemoglobin response to OMONTYS therapy [see DOSAGE AND ADMINISTRATION].
Contact Affymax, Inc. (1-855-466-6689) to perform assays for binding and neutralizing antibodies.
Dialysis Management
Patients may require adjustments in their dialysis prescriptions after initiation of OMONTYS. Patients receiving OMONTYS may require increased anticoagulation with heparin to prevent clotting of the extracorporeal circuit during hemodialysis.
Laboratory Monitoring
Evaluate transferrin saturation and serum ferritin prior to and during OMONTYS treatment. Administer supplemental iron therapy when serum ferritin is less than 100 mcg/L or when serum transferrin saturation is less than 20% [see DOSAGE AND ADMINISTRATION]. The majority of patients with CKD will require supplemental iron during the course of ESA therapy. Following initiation of therapy and after each dose adjustment, monitor hemoglobin every 2 weeks until the hemoglobin is stable and sufficient to minimize the need for RBC transfusion. Thereafter, hemoglobin should be monitored at least monthly provided hemoglobin levels remain stable.
Patient Counseling Information
See FDA-approved patient labeling (Medication Guide).
Prior to treatment, inform patients of the risks and benefits of OMONTYS.
Inform patients:
- To read the Medication Guide and to review and discuss any questions or concerns with their healthcare provider before starting OMONTYS and at regular intervals while receiving OMONTYS.
- Of the increased risks of mortality, serious cardiovascular reactions, thromboembolic reactions, stroke, and tumor progression [see WARNINGS AND PRECAUTIONS].
- To undergo regular blood pressure monitoring, adhere to prescribed anti-hypertensive regimen and follow recommended dietary restrictions.
- To seek medical care immediately if they experience any symptoms of an allergic reaction with use of OMONTYS [see WARNINGS AND PRECAUTIONS].
- To contact their healthcare provider for new-onset neurologic symptoms or change in seizure frequency.
- Of the need to have regular laboratory tests for hemoglobin.
Administer OMONTYS under the direct supervision of a healthcare provider or, in situations where a patient has been trained to administer OMONTYS at home, provide instruction on the proper use of OMONTYS, including instructions to:
- Carefully review the Medication Guide and the Instructions for Use
- Avoid the reuse of needles, syringes, or unused portions of the OMONTYS single use vials or single use pre-filled syringes and properly dispose of these items
- Always keep a puncture-proof disposal container available for the disposal of used syringes and needles.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
Peginesatide was not carcinogenic in a 2-year study in rats at doses up to 0.25 mg/kg administered every 3 weeks by intravenous injection. This dose is approximately 14% the human exposure (AUC) at a dose of 0.35 mg/kg in patients on dialysis. Peginesatide was not carcinogenic in a 26-week study in rasH2 transgenic mice when administered by intravenous injection at doses of 0.1, 0.25, or 0.5 mg/kg/dose every 3 weeks.
Peginesatide was not mutagenic or clastogenic in the in vitro Ames assay, in vitro mammalian chromosome aberration assay, and an in vivo mouse erythrocyte micronucleus assay.
When peginesatide was administered intravenously to male and female rats at weekly intervals prior to and during mating, and treated rats mated, fertility was reduced at ≥ 0.1 mg/kg (approximately 5% of the dose of 0.35 mg/kg in patients) and was most evident at doses ≥ 1.0 mg/kg of peginesatide. Adverse effects in males included reduced weight of seminal vesicles and prostate. Decreased viable fetuses at ≥ 0.1 mg/kg in females appeared to be due to pre- and post-implantation losses. There was no apparent drug-related effect on estrous cycles or number of corpora lutea.
Use In Specific Populations
Pregnancy
Pregnancy Category C
There are no adequate and well-controlled studies in pregnant women. Peginesatide was teratogenic and caused embryofetal lethality when administered to pregnant animals at doses and/or exposures that resulted in polycythemia. OMONTYS should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Administration of peginesatide by intravenous injection to rats and rabbits during organogenesis was associated with embryofetal toxicity and malformations. Dosing was every third day in rats for a total of 5 doses and every fifth day in rabbits for a total of 3 doses (0.01 to 50 mg/kg/dose). In rats and rabbits, adverse embryofetal effects included reduced fetal weight, increased resorption, embryofetal lethality, cleft palate (rats only), sternum anomalies, unossification of sternebrae and metatarsals, and reduced ossification of some bones. Embryofetal toxicity was evident in rats at peginesatide doses of ≥ 1 mg/kg and the malformations (cleft palate and sternoschisis, and variations in blood vessels) were mostly evident at doses of ≥ 10 mg/kg. The dose of 1 mg/kg results in exposures (AUC) comparable to those in humans after intravenous administration at a dose of 0.35 mg/kg in patients on dialysis. In a separate embryofetal developmental study in rats, reduced fetal weight and reduced ossification were seen at a lower dose of 0.25 mg/kg. Reduced fetal weight and delayed ossification in rabbits were observed at ≥ 0.5 mg/kg/dose of peginesatide. In a separate embryofetal developmental study in rabbits, adverse findings were observed at lower doses and included increased incidence of fused sternebrae at 0.25 mg/kg. The effects in rabbits were observed at doses lower (5% - 50%) than the dose of 0.35 mg/kg in patients.
Nursing Mothers
It is not known whether peginesatide is excreted in human milk. Because many drugs are excreted into human milk, caution should be exercised when OMONTYS is administered to a nursing woman.
Pediatric Use
The safety and efficacy of OMONTYS in pediatric patients have not been established.
Geriatric Use
Of the total number of dialysis patients in Phase 3 clinical studies of OMONTYS, 32.5% were age 65 and over, while 13% were age 75 and over. No overall differences in safety or effectiveness were observed between these subjects and younger subjects.
Overdose Information for Omontys
OMONTYS overdosage can elevate hemoglobin levels above the desired level, which should be managed with discontinuation or reduction of OMONTYS dosage and/or with phlebotomy, as clinically indicated [see Pharmacodynamics]. Cases of severe hypertension have been observed following overdose with ESAs [see WARNINGS AND PRECAUTIONS].
Contraindications for Omontys
OMONTYS is contraindicated in patients with:
- Uncontrolled hypertension [see WARNINGS AND PRECAUTIONS].
- Serious allergic reactions to OMONTYS which may include anaphylaxis [see WARNINGS AND PRECAUTIONS].
Clinical Pharmacology for Omontys
Mechanism of Action
Peginesatide binds to and activates the human erythropoietin receptor and stimulates erythropoiesis in human red cell precursors in vitro.
Pharmacodynamics
Peginesatide increases the reticulocyte count, followed by increases in hemoglobin. The rate of hemoglobin increase varies among patients and is dependent upon the dose of peginesatide administered.
Effect on Cardiac Repolarization
The effect of OMONTYS (0.1 mg/kg intravenously) on QTc interval was evaluated in a randomized, double-blind, double-dummy, three-period crossover thorough QT study in 65 healthy subjects. A dose of 0.1 mg/kg administered intravenously did not delay cardiac repolarization compared to placebo. The dose of 0.1 mg/kg is adequate to represent the median dose (0.07 mg/kg) in the phase 3 trials, however is not sufficient to represent doses higher than 0.1 mg/kg in the intended patients.
Pharmacokinetics
Following single intravenous and subcutaneous injections at doses ranging from 0.03 to 0.1 mg/kg to dialysis patients, maximal plasma concentration (Cmax) and area under the plasma concentration versus time curve (AUC) increase with dose. Following subcutaneous administration, the maximum concentrations of peginesatide are reached in approximately 48 hours. The bioavailability of peginesatide following subcutaneous administration is approximately 46%.
No mass balance study has been conducted in humans. Preclinical radiolabeled peginesatide study indicated that peginesatide is not metabolized and that urinary excretion was the predominant route of elimination following either intravenous or subcutaneous dosing.
The mean half-life is 25.0 ± 7.6 hours following intravenous administration and 53.0 ± 17.7 hours following subcutaneous administration in healthy subjects. The mean half-life in dialysis patients is 47.9 ± 16.5 hours following intravenous administration. Mean systemic clearance is 0.5 ± 0.2 mL/hr·kg and mean volume of distribution is 34.9 ± 13.8 mL/kg following intravenous administration in dialysis patients. No accumulation is observed following administration every 4 weeks following intravenous or subcutaneous administration. The pharmacokinetics of peginesatide in patients with CKD on dialysis are not altered by age, gender or race based on population pharmacokinetic analyses.
Clinical Studies
The efficacy and safety of OMONTYS in patients with CKD on dialysis were demonstrated in two randomized, active-controlled, open-label, multi-center clinical studies that evaluated OMONTYS in the maintenance of hemoglobin concentrations in patients who were being treated with another ESA (epoetin alfa or epoetin beta) at the time of study entry. The primary efficacy endpoint for each study was the change in hemoglobin from Baseline to the Evaluation Period (Weeks 29 to 36) using a non-inferiority comparison with epoetin. In Study 1, patients received epoetin via the intravenous route of administration and continued to use this route after randomization to either OMONTYS or epoetin. The average patient exposure year [PEY] per patient was 1.14 years for OMONTYS and the average PEY per patient was 1.25 years for epoetin. In Study 2, the route of administration previously used for epoetin (intravenous or subcutaneous) was used. The average PEY per patient was 1.17 years for OMONTYS and the average PEY per patient was 1.16 years for epoetin. The median dose of OMONTYS was 0.07 mg/kg administered once monthly and the median dose of epoetin was 113 units/kg administered weekly (in 1 to 3 doses).
Patients were randomized (2 to 1) to receive OMONTYS once monthly, or to continue on their current ESA treatment 1 to 3 times per week. The OMONTYS starting dose was based on the patient's total weekly ESA dose during the last week of the screening period. As shown in Table 4, treatment with OMONTYS once monthly and treatment with epoetin 1-3 times per week maintained hemoglobin concentrations within the study pre-specified hemoglobin range (10 to 12 g/dL). In both studies, the proportion of patients receiving transfusions was similar in each treatment group.
Table 4: Clinical Studies in Dialysis Patients
| Group (N) | Mean baseline Hemoglobin | Change from Baseline to Weeks 29-36 | Between group difference, Least Squares Mean g/dL (95% CI) |
| Study 1 | |||
| OMONTYS (524) | 11.3 g/dL | -0.24 g/dL | -0.15 g/dL (-0.30, -0.01) |
| Epoetin (269) | 11.3 g/dL | -0.09 g/dL | |
| Study 2 | |||
| OMONTYS (542) | 11.2 g/dL | -0.07 g/dL | 0.10 g/dL (-0.05, 0.26) |
| Epoetin (273) | 11.2 g/dL | -0.17 g/dL | |
Studies 1 and 2 had a pre-specified, prospective, pooled analysis of a composite cardiovascular safety endpoint consisting of death, myocardial infarction, stroke, or serious adverse events of congestive heart failure, unstable angina or arrhythmia. In patients receiving OMONTYS, 22.8% experienced one of these events compared to 24.4% receiving epoetin (hazard ratio 0.95, 95% CI 0.77, 1.17).
Patient Information for Omontys
MEDICATION GUIDE
OMONTYS®
(O-mon-tis)
(peginesatide) Injection
Read this Medication Guide:
- before you start OMONTYS.
- if you are told by your healthcare provider that there is new information about OMONTYS.
- if you are told by your healthcare provider that you may inject OMONTYS at home, read this Medication Guide each time you receive a new supply of medicine.
This Medication Guide does not take the place of talking to your healthcare provider about your medical condition or your treatment. Talk with your healthcare provider regularly about the use of OMONTYS and ask if there is new information about OMONTYS.
What is the most important information I should know about OMONTYS?
Using OMONTYS or other erythropoiesis-stimulating agents (ESAs) can lead to serious side effects including death.
- If you decide to take OMONTYS, your healthcare provider should prescribe the smallest dose of OMONTYS that is needed to reduce your chance of needing red blood cell transfusions.
- You may get serious heart problems such as heart attack, stroke, heart failure, and may die sooner if you are treated with OMONTYS to reach a normal or near-normal hemoglobin level.
- You may get blood clots while receiving OMONTYS. If you are receiving OMONTYS and you are going to have surgery, talk to your healthcare provider about whether or not you need to take a blood thinner to lessen the chance of blood clots during or following surgery. Clots can form in blood vessels (veins), especially in your leg (deep venous thrombosis or DVT). Pieces of a blood clot may travel to the lungs and block the blood circulation in the lungs (pulmonary embolus).
Call your healthcare provider or get medical help right away if you have any of these symptoms of blood clots:
- Chest pain
- Trouble breathing or shortness of breath
- Pain in the legs, with or without swelling
- A cool or pale arm or leg
- Sudden confusion, trouble speaking, or understanding others' speech
- Sudden numbness or weakness of the face, arm, or leg, especially on one side of your body
- Sudden trouble seeing
- Sudden trouble walking, dizziness, loss of balance or coordination
- Loss of consciousness (fainting)
- Hemodialysis vascular access stops working.
See “What are the possible side effects of OMONTYS?” below.
What is OMONTYS?
OMONTYS is a prescription medicine that works like the human protein erythropoietin. OMONTYS is given to treat anemia (low red blood cells) in adults with chronic kidney disease (CKD) who are on dialysis.
OMONTYS stimulates your bone marrow to make more red blood cells. Having more red blood cells raises your hemoglobin level. If your hemoglobin level stays too high or if your hemoglobin goes up too quickly, this may lead to serious health problems which may result in death. These serious health problems may happen even if you take OMONTYS and do not have an increase in your hemoglobin level.
OMONTYS should not be used for the treatment of anemia:
- if you have chronic kidney disease (CKD) and are not on dialysis,
- if you are receiving treatment for cancer and your anemia is not caused by CKD,
- in place of emergency treatment for anemia (red blood cell transfusions).
OMONTYS has not been proven to improve the quality of life, fatigue, or well-being.
It is not known if OMONTYS is safe and effective in children.
Who should not use OMONTYS?
Do not use OMONTYS if you:
- have high blood pressure that is not controlled (uncontrolled hypertension).have had a serious allergic reaction to OMONTYS.
What should I tell my healthcare provider before using OMONTYS?
Before using OMONTYS tell your healthcare provider if you:
- have heart disease
- have or develop cancer
- have high blood pressure
- have any history of stroke, blood clot or seizure (convulsion)
- have blood disorders (such as sickle cell anemia or clotting disorders)
- have any other medical conditions
- are pregnant or plan to become pregnant. It is not known if OMONTYS will harm your unborn baby.
- are breastfeeding or plan to breastfeed. It is not known if OMONTYS passes into your breast milk.
Tell your healthcare provider about all of the medicines you take, including prescription and non-prescription medicines, vitamins, and herbal supplements.
Know the medicines you take. Keep a list of your medicines with you and show it to your healthcare provider when you get a new medicine.
How should I use OMONTYS?
- Continue to follow your healthcare provider's instructions for diet, dialysis, and medicines, including medicines for high blood pressure, while using OMONTYS.
- Have your blood pressure checked as instructed by your healthcare provider.
- Your healthcare provider should do blood tests to check your hemoglobin and iron levels before and during your treatment with OMONTYS.
- OMONTYS is given one time a month into the vein, through your hemodialysis vascular access (intravenous), or under your skin (subcutaneous).
- OMONTYS should be given by your healthcare provider. In some cases, your healthcare provider may allow you or your caregiver to give the injections at home.
- If you or your caregiver has been trained to give the
injections at home, it is important that you:
- Carefully follow the instructions that your healthcare provider gives you. Be sure that you read, understand, and follow the “Instructions for Use” that come with OMONTYS.
- Use OMONTYS exactly as your healthcare provider tells you to. Do not change the dose of OMONTYS unless told to do so by your healthcare provider.
- Your healthcare provider will show you or your caregiver how much OMONTYS to use, how to inject it, how often it should be injected and how to safely dispose of the used containers, needles and syringes.
- If you miss a dose of OMONTYS, call your healthcare provider right away for instructions on what to do.
- If you inject more than the prescribed amount of OMONTYS, call your healthcare provider right away for instructions on what to do.
What are the possible side effects of OMONTYS?
OMONTYS may cause serious side effects, including:
- See “What is the most important information I should know about OMONTYS?”
- High blood pressure. High blood pressure is a common side effect of OMONTYS in people with chronic kidney disease. Your blood pressure may go up or be difficult to control with blood pressure medicine while taking OMONTYS. This can happen even if you have never had high blood pressure before. Your healthcare provider should check your blood pressure often. If your blood pressure does increase, your healthcare provider may prescribe new or more blood pressure medicine.
- Serious allergic reactions. Serious allergic reactions can cause dizziness or fainting because of a drop in blood pressure, trouble breathing, tightness in the chest, swelling around your mouth, tongue or face, or itching all over your body. If you have a serious allergic reaction, stop using OMONTYS and call your healthcare provider or get emergency medical help right away.
- Antibodies to OMONTYS. Your body may make antibodies to OMONTYS. These antibodies can block or lessen your body's ability to make red blood cells and cause you to have severe anemia. Call your healthcare provider if you have unusual tiredness, lack of energy, dizziness, or fainting. You may need to stop taking OMONTYS.
The most common side effects of OMONTYS include:
- shortness of breath
- diarrhea
- nausea
- vomiting
- cough
- problems with your hemodialysis access
- headache
- muscle spasms
- joint, back, leg, or arm pain
- low blood pressure (hypotension)
- fever
- increase in blood potassium level
- upper respiratory infection
Tell your healthcare provider if you have any side effect that bothers you or that does not go away.
These are not all the possible side effects of OMONTYS. For more information, ask your healthcare provider or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store OMONTYS?
- Keep OMONTYS in the original package.
- Protect OMONTYS from light.
- Store OMONTYS in the refrigerator between 36 °F to 46 °F (2 °C to 8 °C).
- If a refrigerator is not available, OMONTYS vials and pre-filled syringes can be stored at 77 °F or less (25 °C or less) for up to 30 days.
- Throw away multiple use vials of OMONTYS no later than 28 days from the first day that you put a needle into the vial.
- OMONTYS single use vials and pre-filled syringes should be used only one time. Dispose of the single use vial and the pre-filled syringe after use even if there is medicine left in the container.
Keep OMONTYS and all medicines out of the reach of children.
General Information about the safe and effective use of OMONTYS
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use OMONTYS for a condition for which it was not prescribed. Do not give OMONTYS to other people, even if they have the same symptoms that you have. It may harm them.
This Medication Guide summarizes the most important information about OMONTYS. If you would like more information about OMONTYS, talk with your healthcare provider. You can ask your healthcare provider or pharmacist for information about OMONTYS that is written for healthcare professionals.
For more information go to www.omontys.com or call 1-855-466-6689.
What are the ingredients in OMONTYS?
Active ingredient: peginesatide
Inactive ingredients:
- Single Use Vials and Single Use Pre-filled Syringes: sorbitol, sodium phosphate monobasic (dihydrate), sodium phosphate dibasic, polysorbate 20, and sodium hydroxide in Water for Injection.
- Multiple Use Vials: sorbitol, phenol, L-methionine, glacial acetic acid, and sodium hydroxide in Water for Injection.
This Medication Guide has been approved by the U.S. Food and Drug Administration.
From 
Healthy Resources

Report Problems to the Food and Drug Administration
You are encouraged to report negative side effects of prescription drugs to the FDA. Visit the FDA MedWatch website or call 1-800-FDA-1088.