Description for Phyrago
PHYRAGO (dasatinib) is a kinase inhibitor. The chemical name for dasatinib (anhydrous) is N(2-chloro-6-methylphenyl)-2-[[6-[4-(2-hydroxyethyl)-1-piperazinyl]-2-methyl-4pyrimidinyl]amino]-5-thiazolecarboxamide. The molecular formula is C22H26ClN7O2S, which
corresponds to a formula weight of 488.01. Dasatinib (anhydrous) has the following chemical structure:
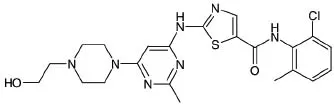 |
Dasatinib (anhydrous) is a white to light yellow powder. The drug substance is insoluble in water and slightly soluble in ethanol and methanol.
PHYRAGO is supplied as white to light yellow, biconvex, immediate release tablets for oral use containing dasatinib (anhydrous), with the following inactive ingredients: croscarmellose sodium, dibasic calcium phosphate, magnesium stearate, methacrylic acid-ethyl acrylate copolymer, microcrystalline cellulose, propyl gallate, and silica dimethyl silylate.
Uses for Phyrago
PHYRAGO is indicated for the treatment of adult patients with
- newly diagnosed Philadelphia chromosome-positive (Ph+) chronic myeloid leukemia (CML) in chronic phase.
- chronic, accelerated, or myeloid or lymphoid blast phase Ph+ CML with resistance or intolerance to prior therapy including imatinib.
- Philadelphia chromosome-positive acute lymphoblastic leukemia (Ph+ ALL) with resistance or intolerance to prior therapy.
Pediatric use information is approved for Bristol-Myers Squibb Company’s SPRYCEL (dasatinib) tablets. However, due to Bristol-Myers Squibb Company’s marketing exclusivity rights, this drug product is not labeled with that pediatric information.
Dosage for Phyrago
Recommended Dosage In Adult Patients
The recommended starting dosage of PHYRAGO for chronic phase CML in adults is 100 mg administered orally once daily. The recommended starting dosage of PHYRAGO for accelerated phase CML, myeloid or lymphoid blast phase CML, or Ph+ ALL in adults is 140 mg administered orally once daily. Swallow PHYRAGO whole. Do not crush, cut, or chew the tablets. PHYRAGO can be taken with or without a meal, either in the morning or in the evening.
Dosage Modifications
Strong CYP3A4 Inducers
Avoid the use of concomitant strong CYP3A4 inducers. If patients must be coadministered a strong CYP3A4 inducer, consider a PHYRAGO dose increase. If the dose of PHYRAGO is increased, monitor the patient carefully for toxicity [see DRUG INTERACTIONS].
Strong CYP3A4 Inhibitors
Avoid the use of concomitant strong CYP3A4 inhibitors and grapefruit juice. Recommend selecting an alternate concomitant medication with no or minimal enzyme inhibition potential, if possible. If PHYRAGO must be administered with a strong CYP3A4 inhibitor, consider a dose decrease to:
- 40 mg daily for patients taking PHYRAGO 140 mg daily.
- 20 mg daily for patients taking PHYRAGO 100 mg daily.
- 20 mg daily for patients taking PHYRAGO 70 mg daily.
For patients taking PHYRAGO 60 mg or 40 mg daily, consider interrupting PHYRAGO until the inhibitor is discontinued. Allow a washout period of approximately 1 week after the inhibitor is stopped before reinitiating PHYRAGO.
These reduced doses of PHYRAGO are predicted to adjust the area under the curve (AUC) to the range observed without CYP3A4 inhibitors; however, clinical data are not available with these dose adjustments in patients receiving strong CYP3A4 inhibitors. If PHYRAGO is not tolerated after dose reduction, either discontinue the strong CYP3A4 inhibitor or interrupt PHYRAGO until the inhibitor is discontinued. Allow a washout period of approximately 1 week after the inhibitor is stopped before the PHYRAGO dose is increased [see DRUG INTERACTIONS].
Dose Escalation In Adults With CML And Ph+ ALL
For adult patients with CML and Ph+ ALL, consider dose escalation to 140 mg once daily (chronic phase CML) or 180 mg once daily (advanced phase CML and Ph+ ALL) in patients who do not achieve a hematologic or cytogenetic response at the recommended starting dosage.
Dosage Adjustment For Adverse Reactions
Myelosuppression
In clinical studies, myelosuppression was managed by dose interruption, dose reduction, or discontinuation of study therapy. Hematopoietic growth factor has been used in patients with resistant myelosuppression. Guidelines for dose modifications for adult patients are summarized in Table 1.
Table 1: Dosage Adjustments for Neutropenia and Thrombocytopenia in Adults
| Chronic Phase CML (starting dose 100 mg once daily) |
ANC* <0.5 x 109/L or Platelets <50 x 109/L |
|
| Accelerated Phase CML, Blast Phase CML and Ph+ ALL (starting dose 140 mg once daily) |
ANC* <0.5 x 109/L or Platelets <10 x 109/L |
|
| *ANC: absolute neutrophil count | ||
Non-Hematologic Adverse Reactions
For adults with Ph+ CML and ALL, if a severe non-hematologic adverse reaction develops with PHYRAGO use, treatment must be withheld until the adverse reaction has resolved or improved. Thereafter, treatment can be resumed as appropriate at a reduced dose depending on the severity and recurrence [see WARNINGS AND PRECAUTIONS].
Duration Of Treatment
In clinical studies, treatment with dasatinib in adults with chronic phase CML was continued until disease progression or until no longer tolerated by the patient. The effect of stopping treatment on long-term disease outcome after the achievement of a cytogenetic response (including complete cytogenetic response [CCyR]) or major molecular response (MMR and MR4.5) has not been established.
PHYRAGO is a hazardous product. Follow applicable special handling and disposal procedures.1
Pediatric use information is approved for Bristol-Myers Squibb Company’s SPRYCEL (dasatinib) tablets. However, due to Bristol-Myers Squibb Company’s marketing exclusivity rights, this drug product is not labeled with that pediatric information.
HOW SUPPLIED
Dosage Forms And Strengths
PHYRAGO is available as 20 mg, 50 mg, 70 mg, 80 mg, 100 mg, and 140 mg white to light yellow, biconvex, immediate release tablets.
Storage And Handling
PHYRAGO (dasatinib) tablets are available in bottles with a child-resistant closure and desiccant container(s) as described in Table 13. The desiccant container(s) should remain within the bottle after opening and should not be swallowed or eaten.
Table 13: PHYRAGO Trade Presentations
| NDC Number | Strength | Description | Tablets per Bottle |
| xxxx-xxxx-xx | 20 mg | white to light-yellow, biconvex, round shaped tablet with “NC 2” debossed on one side and plain on the other side | 60 |
| xxxx-xxxx-xx | 50 mg | white to light-yellow, biconvex, oval shaped tablet with “NC 5” debossed on one side and plain on the other side | 60 |
| xxxx-xxxx-xx | 70 mg | white to light-yellow, biconvex, round shaped tablet with “NC 7” debossed on one side and plain on the other side | 60 |
| xxxx-xxxx-xx | 80 mg | white to light-yellow, biconvex, triangular shaped tablet with “NC 8” debossed on one side and plain on the other side | 30 |
| xxxx-xxxx-xx | 100mg | white to light-yellow, biconvex, oval shaped tablet with “NC 10” debossed on one side and plain on the other side | 30 |
| xxxx-xxxx-xx | 140mg | white to light-yellow, biconvex, round shaped tablet with “NC 14” debossed on one side and plain on the other side | 30 |
Storage
PHYRAGO should be stored at 20°C to 25°C (68°F to 77°F); excursions permitted between 15°C and 30°C (59°F and 86°F) [see USP Controlled Room Temperature].
Handling And Disposal
PHYRAGO is a hazardous product. Follow special handling and disposal procedures.1
Personnel who are pregnant should avoid exposure to tablets.
To prevent exposure of healthcare professionals to the active substance, the use of latex or nitrile gloves for appropriate disposal when handling tablets is recommended, to minimize the risk of dermal exposure.
REFERENCES
1. http://www.osha.gov/SLTC/hazardousdrugs/index.html
Distributed by: Nanocopoeia, LLC, 639 Campus Dr, New Brighton, MN 55112. Revised: Dec 2023.
Side Effects for Phyrago
The following clinically significant adverse reactions are discussed in greater detail in other sections of the labeling:
- Myelosuppression [see DOSAGE AND ADMINISTRATION and WARNINGS AND PRECAUTIONS].
- Bleeding-related events [see WARNINGS AND PRECAUTIONS].
- Fluid retention [see WARNINGS AND PRECAUTIONS].
- Cardiovascular toxicity [see WARNINGS AND PRECAUTIONS].
- Pulmonary arterial hypertension [see WARNINGS AND PRECAUTIONS].
- QT prolongation [see WARNINGS AND PRECAUTIONS].
- Severe dermatologic reactions [see WARNINGS AND PRECAUTIONS].
- Tumor lysis syndrome [see WARNINGS AND PRECAUTIONS].
- Effects on growth and development in pediatric patients [see WARNINGS AND PRECAUTIONS].
- Hepatotoxicity [see WARNINGS AND PRECAUTIONS].
Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The data described below reflect exposure to dasatinib administered as single-agent therapy at all doses tested in clinical studies, including 324 adult patients with newly diagnosed chronic phase CML and 2388 adult patients with imatinib-resistant or -intolerant chronic or advanced phase CML or Ph+ ALL. The median duration of therapy in a total of 2712 adult patients was 19.2 months (range 0 to 93.2 months). In a randomized trial in patients with newly diagnosed chronic phase CML, the median duration of therapy was approximately 60 months. The median duration of therapy in 1618 adult patients with chronic phase CML was 29 months (range 0 to 92.9 months).
The median duration of therapy in 1094 adult patients with advanced phase CML or Ph+ ALL was 6.2 months (range 0 to 93.2 months).
In the overall population of 2712 adult patients, 88% of patients experienced adverse reactions at some time and 19% experienced adverse reactions leading to treatment discontinuation.
In the randomized trial in adult patients with newly diagnosed chronic phase CML, drug was discontinued for adverse reactions in 16% of patients with a minimum of 60 months of followup. After a minimum of 60 months of follow-up, the cumulative discontinuation rate was 39%. Among the 1618 patients with chronic phase CML, drug-related adverse reactions leading to discontinuation were reported in 329 (20.3%) patients; among the 1094 patients with advanced phase CML or Ph+ ALL, drug-related adverse reactions leading to discontinuation were reported in 191 (17.5%) patients.
Adverse reactions reported in ≥10% of adult patients, and other adverse reactions of interest, in a randomized trial in patients with newly diagnosed chronic phase CML at a median follow-up of approximately 60 months are presented in Table 2.
Adverse reactions reported in ≥10% of adult patients treated at the recommended dose of 100 mg once daily (n=165), and other adverse reactions of interest, in a randomized dose-optimization trial of patients with chronic phase CML resistant or intolerant to prior imatinib therapy at a median follow-up of approximately 84 months are presented in Table 4.
Drug-related serious adverse reactions (SARs) were reported for 16.7% of adult patients in the randomized trial of patients with newly diagnosed chronic phase CML. Serious adverse reactions reported in ≥5% of patients included pleural effusion (5%).
Drug-related SARs were reported for 26.1% of patients treated at the recommended dose of 100 mg once daily in the randomized dose-optimization trial of adult patients with chronic phase CML resistant or intolerant to prior imatinib therapy. Serious adverse reactions reported in ≥5% of patients included pleural effusion (10%).
Chronic Myeloid Leukemia (CML)
Adverse reactions (excluding laboratory abnormalities) that were reported in at least 10% of adult patients are shown in Table 2 for newly diagnosed patients with chronic phase CML and Tables 4 and 6 for CML patients with resistance or intolerance to prior imatinib therapy.
Table 2: Adverse Reactions Reported in ≥10% of Adult Patients with Newly Diagnosed Chronic Phase CML (Minimum of 60 Months Follow-up)
| All Grades | Grade 3/4 | |||
| Dasatinib (n=258) | Imatinib (n=258) | Dasatinib (n=258) | Imatinib (n=258) | |
| Adverse Reaction | Percent (%) of Patients | |||
| Fluid retention | 38 | 45 | 5 | 1 |
| Pleural effusion | 28 | 1 | 3 | 0 |
| Superficial localized edema | 14 | 38 | 0 | <1 |
| Pulmonary hypertension | 5 | <1 | 1 | 0 |
| Generalized edema | 4 | 7 | 0 | 0 |
| Pericardial effusion | 4 | 1 | 1 | 0 |
| Congestive heart failure/ Cardiac dysfunctiona | 2 | 1 | <1 | <1 |
| Pulmonary edema | 1 | 0 | 0 | 0 |
| Diarrhea | 22 | 23 | 1 | 1 |
| Musculoskeletal pain | 14 | 17 | 0 | <1 |
| Rashb | 14 | 18 | 0 | 2 |
| Headache | 14 | 11 | 0 | 0 |
| Abdominal pain | 11 | 8 | 0 | 1 |
| Fatigue | 11 | 12 | <1 | 0 |
| Nausea | 10 | 25 | 0 | 0 |
| Myalgia | 7 | 12 | 0 | 0 |
| Arthralgia | 7 | 10 | 0 | <1 |
| Hemorrhagec | 8 | 8 | 1 | 1 |
| Gastrointestinal bleeding | 2 | 2 | 1 | 0 |
| Other bleedingd | 6 | 6 | 0 | <1 |
| CNS bleeding | <1 | <1 | 0 | <1 |
| Vomiting | 5 | 12 | 0 | 0 |
| Muscle spasms | 5 | 21 | 0 | <1 |
| a Includes cardiac failure acute, cardiac failure congestive, cardiomyopathy, diastolic dysfunction, ejection fraction decreased, and left ventricular dysfunction. b Includes erythema, erythema multiforme, rash, rash generalized, rash macular, rash papular, rash pustular, skin exfoliation, and rash vesicular. c Adverse reaction of special interest with <10% frequency. d Includes conjunctival hemorrhage, ear hemorrhage, ecchymosis, epistaxis, eye hemorrhage, gingival bleeding, hematoma, hematuria, hemoptysis, intra-abdominal hematoma, petechiae, scleral hemorrhage, uterine hemorrhage, and vaginal hemorrhage. |
||||
A comparison of cumulative rates of adverse reactions reported in ≥10% of patients with minimum follow-up of 1 and 5 years in a randomized trial of newly diagnosed patients with chronic phase CML treated with dasatinib are shown in Table 3.
Table 3: Adverse Reactions Reported in ≥10% of Adult Patients with Newly Diagnosed Chronic Phase CML in the Dasatinib-Treated Arm (n=258)
| Minimum of 1 Year Follow-up | Minimum of 5 Years Follow-up | |||
| All Grades | Grade 3/4 | All Grades | Grade 3/4 | |
| Adverse Reaction | Percent (%) of Patients | |||
| Fluid retention | 19 | 1 | 38 | 5 |
| Pleural effusion | 10 | 0 | 28 | 3 |
| Superficial localized edema | 9 | 0 | 14 | 0 |
| Pulmonary hypertension | 1 | 0 | 5 | 1 |
| Generalized edema | 2 | 0 | 4 | 0 |
| Pericardial effusion | 1 | <1 | 4 | 1 |
| Congestive heart failure/cardiac dysfunctiona | 2 | <1 | 2 | <1 |
| Pulmonary edema | <1 | 0 | 1 | 0 |
| Diarrhea | 17 | <1 | 22 | 1 |
| Musculoskeletal pain | 11 | 0 | 14 | 0 |
| Rashb | 11 | 0 | 14 | 0 |
| Headache | 12 | 0 | 14 | 0 |
| Abdominal pain | 7 | 0 | 11 | 0 |
| Fatigue | 8 | <1 | 11 | 0 |
| Nausea | 8 | 0 | 10 | 0 |
| a Includes cardiac failure acute, cardiac failure congestive, cardiomyopathy, diastolic dysfunction, ejection fraction decreased, and left ventricular dysfunction. b Includes erythema, erythema multiforme, rash, rash generalized, rash macular, rash papular, rash pustular, skin exfoliation, and rash vesicular. |
||||
At 60 months, there were 26 deaths in dasatinib-treated patients (10.1%) and 26 deaths in imatinib-treated patients (10.1%); 1 death in each group was assessed by the investigator as related to study therapy.
Table 4: Adverse Reactions Reported in ≥10% of Adult Patients with Chronic Phase CML Resistant or Intolerant to Prior Imatinib Therapy (minimum of 84 months follow-up)
| 100 mg Once Daily | ||
| Chronic (n=165) | ||
| All Grades | Grade 3/4 | |
| Adverse Reaction | Percent (%) of Patients | |
| Fluid retention | 48 | 7 |
| Superficial localized edema | 22 | 0 |
| Pleural effusion | 28 | 5 |
| Generalized edema | 4 | 0 |
| Pericardial effusion | 3 | 1 |
| Pulmonary hypertension | 2 | 1 |
| Headache | 33 | 1 |
| Diarrhea | 28 | 2 |
| Fatigue | 26 | 4 |
| Dyspnea | 24 | 2 |
| Musculoskeletal pain | 22 | 2 |
| Nausea | 18 | 1 |
| Skin rasha | 18 | 2 |
| Myalgia | 13 | 0 |
| Arthralgia | 13 | 1 |
| Infection (including bacterial, viral, fungal, and non-specified) | 13 | 1 |
| Abdominal pain | 12 | 1 |
| Hemorrhage | 12 | 1 |
| Gastrointestinal bleeding | 2 | 1 |
| Pruritus | 12 | 1 |
| Pain | 11 | 1 |
| Constipation | 10 | 1 |
| a Includes drug eruption, erythema, erythema multiforme, erythrosis, exfoliative rash, generalized erythema, genital rash, heat rash, milia, rash, rash erythematous, rash follicular, rash generalized, rash macular, rash maculopapular, rash papular, rash pruritic, rash pustular, skin exfoliation, skin irritation, urticaria vesiculosa, and rash vesicular. | ||
Cumulative rates of selected adverse reactions that were reported over time in patients treated with the 100 mg once daily recommended starting dose in a randomized dose-optimization trial of imatinib-resistant or -intolerant patients with chronic phase CML are shown in Table 5.
Table 5: Selected Adverse Reactions Reported in Adult Dose Optimization Trial (Imatinib-intolerant or -Resistant Chronic Phase CML)a
| Minimum of 2 Years Follow-up | Minimum of 5 Years Followup | Minimum of 7 Years Follow-up | ||||
| All Grades | Grade 3/4 | All Grades | Grade 3/4 | All Grades | Grade 3/4 | |
| Adverse Reaction | Percent (%) of Patients | |||||
| Diarrhea | 27 | 2 | 28 | 2 | 28 | 2 |
| Fluid retention | 34 | 4 | 42 | 6 | 48 | 7 |
| Superficial edema | 18 | 0 | 21 | 0 | 22 | 0 |
| Pleural effusion | 18 | 2 | 24 | 4 | 28 | 5 |
| Generalized edema | 3 | 0 | 4 | 0 | 4 | 0 |
| Pericardial effusion | 2 | 1 | 2 | 1 | 3 | 1 |
| Pulmonary hypertension | 0 | 0 | 0 | 0 | 2 | 1 |
| Hemorrhage | 11 | 1 | 11 | 1 | 12 | 1 |
| Gastrointestinal bleeding | 2 | 1 | 2 | 1 | 2 | 1 |
| a Randomized dose-optimization trial results reported in the recommended starting dose of 100 mg once daily (n=165) population. | ||||||
Table 6: Adverse Reactions Reported in ≥10% of Adult Patients with Advanced Phase CML Resistant or Intolerant to Prior Imatinib Therapy
| 140 mg Once Daily | ||||||
| Accelerated (n=157) |
Myeloid Blast (n=74) |
Lymphoid Blast (n=33) |
||||
| All Grades | Grade 3/4 | All Grades | Grade 3/4 | All Grades | Grade 3/4 | |
| Adverse Reaction | Percent (%) of Patients | |||||
| Fluid retention | 35 | 8 | 34 | 7 | 21 | 6 |
| Superficial localized edema | 18 | 1 | 14 | 0 | 3 | 0 |
| Pleural effusion | 21 | 7 | 20 | 7 | 21 | 6 |
| Generalized edema | 1 | 0 | 3 | 0 | 0 | 0 |
| Pericardial effusion | 3 | 1 | 01 | 0 | 0 | 0 |
| Congestive heart failure/cardiac dysfunctiona | 0 | 0 | 4 | 0 | 0 | 0 |
| Pulmonary edema | 1 | 0 | 4 | 3 | 0 | 0 |
| Headache | 27 | 1 | 18 | 1 | 15 | 3 |
| Diarrhea | 31 | 3 | 20 | 5 | 18 | 0 |
| Fatigue | 19 | 2 | 20 | 1 | 9 | 3 |
| Dyspnea | 20 | 3 | 15 | 3 | 3 | 3 |
| Musculoskeletal pain | 11 | 0 | 8 | 1 | 0 | 0 |
| Nausea | 19 | 1 | 23 | 1 | 21 | 3 |
| Skin rashb | 15 | 0 | 16 | 1 | 21 | 0 |
| Arthralgia | 10 | 0 | 5 | 1 | 0 | 0 |
| Infection (including bacterial, viral, fungal, and non-specified) | 10 | 6 | 14 | 7 | 9 | 0 |
| Hemorrhage | 26 | 8 | 19 | 9 | 24 | 9 |
| Gastrointestinal bleeding | 8 | 6 | 9 | 7 | 9 | 3 |
| CNS bleeding | 1 | 1 | 0 | 0 | 3 | 3 |
| Vomiting | 11 | 1 | 12 | 0 | 15 | 0 |
| Pyrexia | 11 | 2 | 18 | 3 | 6 | 0 |
| Febrile neutropenia | 4 | 4 | 12 | 12 | 12 | 12 |
| a Includes ventricular dysfunction, cardiac failure, cardiac failure congestive, cardiomyopathy, congestive cardiomyopathy, diastolic dysfunction, ejection fraction decreased, and ventricular failure. b Includes drug eruption, erythema, erythema multiforme, erythrosis, exfoliative rash, generalized erythema, genital rash, heat rash, milia, rash, rash erythematous, rash follicular, rash generalized, rash macular, rash maculopapular, rash papular, rash pruritic, rash pustular, skin exfoliation, skin irritation, urticaria vesiculosa, and rash vesicular. |
||||||
Adverse reactions associated with bone growth and development were reported in 5 (5.2%) of pediatric patients with chronic phase CML [see WARNINGS AND PRECAUTIONS].
Laboratory Abnormalities
Myelosuppression was commonly reported in all patient populations. The frequency of Grade 3 or 4 neutropenia, thrombocytopenia, and anemia was higher in patients with advanced phase CML than in chronic phase CML (Tables 7 and 8). Myelosuppression was reported in patients with normal baseline laboratory values as well as in patients with pre-existing laboratory abnormalities.
In patients who experienced severe myelosuppression, recovery generally occurred following dose interruption or reduction; permanent discontinuation of treatment occurred in 2% of adult patients with newly diagnosed chronic phase CML and 5% of adult patients with resistance or intolerance to prior imatinib therapy [see WARNINGS AND PRECAUTIONS].
Grade 3 or 4 elevations of transaminases or bilirubin and Grade 3 or 4 hypocalcemia, hypokalemia, and hypophosphatemia were reported in patients with all phases of CML but were reported with an increased frequency in patients with myeloid or lymphoid blast phase CML. Elevations in transaminases or bilirubin were usually managed with dose reduction or interruption. Patients developing Grade 3 or 4 hypocalcemia during dasatinib therapy often had recovery with oral calcium supplementation.
Laboratory abnormalities reported in adult patients with newly diagnosed chronic phase CML are shown in Table 7. There were no discontinuations of dasatinib therapy in this patient population due to biochemical laboratory parameters.
Table 7: CTC Grade 3/4 Laboratory Abnormalities in Adult Patients with Newly Diagnosed Chronic Phase CML (Minimum of 60 Months Follow up)
| Dasatinib (n=258) |
Imatinib (n=258) |
|
| Percent (%) of Patients | ||
| Hematology Parameters | ||
| Neutropenia | 29 | 24 |
| Thrombocytopenia | 22 | 14 |
| Anemia | 13 | 9 |
| Biochemistry Parameters | ||
| Hypophosphatemia | 7 | 31 |
| Hypokalemia | 0 | 3 |
| Hypocalcemia | 4 | 3 |
| Elevated SGPT (ALT) | <1 | 2 |
| Elevated SGOT (AST) | <1 | 1 |
| Elevated Bilirubin | 1 | 0 |
| Elevated Creatinine | 1 | 1 |
| CTC grades: neutropenia (Grade 3 ≥0.5 - <1.0 × 109/L, Grade 4 <0.5 × 109/L); thrombocytopenia (Grade 3 ≥25 - <50 × 109/L, Grade 4 <25 × 109/L); anemia (hemoglobin Grade 3 ≥65 - <80 g/L, Grade 4 <65 g/L); elevated creatinine (Grade 3 >3 - 6 × upper limit of normal range (ULN), Grade 4 >6 × ULN); elevated bilirubin (Grade 3 >3 - 10 × ULN, Grade 4 >10 × ULN); elevated SGOT or SGPT (Grade 3 >5 - 20 × ULN, Grade 4 >20 × ULN); hypocalcemia (Grade 3 <7.0 - 6.0 mg/dL, Grade 4 <6.0 mg/dL); hypophosphatemia (Grade 3 <2.0 - 1.0 mg/dL, Grade 4 <1.0 mg/dL); hypokalemia (Grade 3 <3.0 - 2.5 mmol/L, Grade 4 <2.5 mmol/L). | ||
Laboratory abnormalities reported in patients with CML resistant or intolerant to imatinib who received the recommended starting doses of dasatinib are shown by disease phase in Table 8.
Table 8: CTC Grade 3/4 Laboratory Abnormalities in Clinical Studies of CML in Adults: Resistance or Intolerance to Prior Imatinib Therapy
| Chronic Phase CML 100 mg Once Daily | Advanced Phase CML 140 mg Once Daily | |||
| (n=165) | Accelerated Phase (n=157) |
Myeloid Blast Phase (n=74) |
Lymphoid Blast Phase (n=33) |
|
| Percent (%) of Patients | ||||
| Hematology Parameters* | ||||
| Neutropenia | 36 | 58 | 77 | 79 |
| Thrombocytopenia | 24 | 63 | 78 | 85 |
| Anemia | 13 | 47 | 74 | 52 |
| Biochemistry Parameters | ||||
| Hypophosphatemia | 10 | 13 | 12 | 18 |
| Hypokalemia | 2 | 7 | 11 | 15 |
| Hypocalcemia | <1 | 4 | 9 | 12 |
| Elevated SGPT (ALT) | 0 | 2 | 5 | 3 |
| Elevated SGOT (AST) | <1 | 0 | 4 | 3 |
| Elevated Bilirubin | <1 | 1 | 3 | 6 |
| Elevated Creatinine | 0 | 2 | 8 | 0 |
| CTC grades: neutropenia (Grade 3 ≥0.5 - <1.0 × 109/L, Grade 4 <0.5 × 109/L); thrombocytopenia (Grade 3≥25 - <50 × 109/L, Grade 4 <25 × 109/L); anemia (hemoglobin Grade 3 ≥65 - <80 g/L, Grade 4 <65 g/L); elevated creatinine (Grade 3 >3 - 6 × upper limit of normal range (ULN), Grade 4 >6 × ULN); elevated bilirubin (Grade 3 >3 - 10 × ULN, Grade 4 >10 × ULN); elevated SGOT or SGPT (Grade 3 >5 - 20 × ULN, Grade 4 >20 × ULN); hypocalcemia (Grade 3 <7.0 - 6.0 mg/dL, Grade 4 <6.0 mg/dL); hypophosphatemia (Grade 3 <2.0 - 1.0 mg/dL, Grade 4 <1.0 mg/dL); hypokalemia (Grade 3 <3.0 - 2.5 mmol/L, Grade 4 <2.5 mmol/L). *Hematology parameters for 100 mg once-daily dosing in chronic phase CML reflects 60-month minimum followup. |
||||
Among adult patients with chronic phase CML with resistance or intolerance to prior imatinib therapy, cumulative Grade 3 or 4 cytopenias were similar at 2 and 5 years including: neutropenia (36% vs 36%), thrombocytopenia (23% vs 24%), and anemia (13% vs 13%).
Philadelphia Chromosome-Positive Acute Lymphoblastic Leukemia (Ph+ ALL) in Adults
A total of 135 adult patients with Ph+ ALL were treated with dasatinib in clinical studies. The median duration of treatment was 3 months (range 0.03 - 31 months). The safety profile of patients with Ph+ ALL was similar to those with lymphoid blast phase CML. The most frequently reported adverse reactions included fluid retention events, such as pleural effusion (24%) and superficial edema (19%), and gastrointestinal disorders, such as diarrhea (31%), nausea (24%), and vomiting (16%). Hemorrhage (19%), pyrexia (17%), rash (16%), and dyspnea (16%) were also frequently reported. Serious adverse reactions reported in ≥5% of patients included pleural effusion (11%), gastrointestinal bleeding (7%), febrile neutropenia (6%), and infection (5%).
Additional Pooled Data From Clinical Trials
The following additional adverse reactions were reported in patients in dasatinib CML clinical studies and adult patients in Ph+ ALL clinical studies at a frequency of ≥10%, 1% - <10%, 0.1% - <1%, or <0.1%. These adverse reactions are included based on clinical relevance.
Gastrointestinal Disorders: 1% - <10% - mucosal inflammation (including mucositis/stomatitis), dyspepsia, abdominal distension, constipation, gastritis, colitis (including neutropenic colitis), oral soft tissue disorder; 0.1% - <1% - ascites, dysphagia, anal fissure, upper gastrointestinal ulcer, esophagitis, pancreatitis, gastroesophageal reflux disease; <0.1% - protein losing gastroenteropathy, ileus, acute pancreatitis, anal fistula.
General Disorders and Administration-Site Conditions: ≥10% - peripheral edema, face edema; 1% - <10% - asthenia, chest pain, chills; 0.1% - <1% - malaise, other superficial edema, peripheral swelling; <0.1% - gait disturbance.
Skin and Subcutaneous Tissue Disorders: 1% - <10% - alopecia, acne, dry skin, hyperhidrosis, urticaria, dermatitis (including eczema); 0.1% - <1% - pigmentation disorder, skin ulcer, bullous conditions, photosensitivity, nail disorder, neutrophilic dermatosis, panniculitis, palmar-plantar erythrodysesthesia syndrome, hair disorder; <0.1% - leukocytoclastic vasculitis, skin fibrosis.
Respiratory, Thoracic, and Mediastinal Disorders: 1% - <10% - lung infiltration, pneumonitis, cough; 0.1% - <1% - asthma, bronchospasm, dysphonia, pulmonary arterial hypertension; <0.1% - acute respiratory distress syndrome, pulmonary embolism.
Nervous System Disorders: 1% - <10% - neuropathy (including peripheral neuropathy), dizziness, dysgeusia, somnolence; 0.1% - <1% - amnesia, tremor, syncope, balance disorder; <0.1% - convulsion, cerebrovascular accident, transient ischemic attack, optic neuritis, VIIth nerve paralysis, dementia, ataxia.
Blood and Lymphatic System Disorders: 0.1% - <1% - lymphadenopathy, lymphopenia; <0.1% - aplasia pure red cell.
Musculoskeletal and Connective Tissue Disorders: 1% - <10% - muscular weakness, musculoskeletal stiffness; 0.1% - <1% - rhabdomyolysis, tendonitis, muscle inflammation, osteonecrosis, arthritis; <0.1% - epiphyses delayed fusion (reported at 1% - <10% in the pediatric studies), growth retardation (reported at 1% - <10% in the pediatric studies).
Investigations: 1% - <10% - weight increased, weight decreased; 0.1% - <1% - blood creatine phosphokinase increased, gamma-glutamyltransferase increased.
Infections and Infestations: 1% - <10% - pneumonia (including bacterial, viral, and fungal), upper respiratory tract infection/inflammation, herpes virus infection, enterocolitis infection, sepsis (including fatal outcomes [0.2%]).
Metabolism and Nutrition Disorders: 1% - <10% - appetite disturbances, hyperuricemia; 0.1% - <1% - hypoalbuminemia, tumor lysis syndrome, dehydration, hypercholesterolemia; <0.1% - diabetes mellitus.
Cardiac Disorders: 1% - <10% - arrhythmia (including tachycardia), palpitations; 0.1% - <1% - angina pectoris, cardiomegaly, pericarditis, ventricular arrhythmia (including ventricular tachycardia), electrocardiogram T-wave abnormal, troponin increased; <0.1% - cor pulmonale, myocarditis, acute coronary syndrome, cardiac arrest, electrocardiogram PR prolongation, coronary artery disease, pleuropericarditis.
Eye Disorders: 1% - <10% - visual disorder (including visual disturbance, vision blurred, and visual acuity reduced), dry eye; 0.1% - <1% - conjunctivitis, visual impairment, lacrimation increased, <0.1% - photophobia.
Vascular Disorders: 1% - <10% - flushing, hypertension; 0.1% - <1% - hypotension, thrombophlebitis, thrombosis; <0.1% - livedo reticularis, deep vein thrombosis, embolism.
Psychiatric Disorders: 1% - <10% - insomnia, depression; 0.1% - <1% - anxiety, affect lability, confusional state, libido decreased.
Pregnancy, Puerperium, and Perinatal Conditions: <0.1% - abortion.
Reproductive System and Breast Disorders: 0.1% - <1% - gynecomastia, menstrual disorder.
Injury, Poisoning, and Procedural Complications: 1% - <10% - contusion.
Ear and Labyrinth Disorders: 1% - <10% - tinnitus; 0.1% - <1% - vertigo, hearing loss.
Hepatobiliary Disorders: 0.1% - <1% - cholestasis, cholecystitis, hepatitis.
Renal and Urinary Disorders: 0.1% - <1% - urinary frequency, renal failure, proteinuria; <0.1% - renal impairment. Immune System Disorders: 0.1% - <1% - hypersensitivity (including erythema nodosum). Endocrine Disorders: 0.1% - <1% - hypothyroidism; <0.1% - hyperthyroidism, thyroiditis.
Postmarketing Experience
The following adverse reactions have been identified during post approval use of dasatinib. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Infections: hepatitis B virus reactivation
Cardiac disorders: atrial fibrillation/atrial flutter
Respiratory, thoracic, and mediastinal disorders: interstitial lung disease
Skin and subcutaneous tissue disorders: Stevens-Johnson syndrome
Renal and urinary disorders: nephrotic syndrome
Blood and lymphatic system disorders: thrombotic microangiopathy
Hepatobiliary disorders: hepatotoxicity
Pediatric use information is approved for Bristol-Myers Squibb Company’s SPRYCEL (dasatinib) tablets. However, due to Bristol-Myers Squibb Company’s marketing exclusivity rights, this drug product is not labeled with that pediatric information.
Drug Interactions for Phyrago
Effect Of Other Drugs On Dasatinib
Strong CYP3A4 Inhibitors
The coadministration with strong CYP3A inhibitors may increase dasatinib concentrations [see CLINICAL PHARMACOLOGY]. Increased dasatinib concentrations may increase the risk of toxicity. Avoid concomitant use of strong CYP3A4 inhibitors. If concomitant administration of a strong CYP3A4 inhibitor cannot be avoided, consider a dose reduction [see DOSAGE AND ADMINISTRATION].
Strong CYP3A4 Inducers
The coadministration of PHYRAGO with strong CYP3A inducers may decrease dasatinib concentrations [see CLINICAL PHARMACOLOGY]. Decreased dasatinib concentrations may reduce efficacy. Consider alternative drugs with less enzyme induction potential. If concomitant administration of a strong CYP3A4 inducer cannot be avoided, consider a dose increase.
Antacids
Avoid administration of PHYRAGO with antacids. If administration of an antacid cannot be avoided, administer the antacid at least 2 hours prior to or 2 hours after the dose of PHYRAGO.
Concomitant use with antacids decreases dasatinib plasma concentrations [see CLINICAL PHARMACOLOGY], which may reduce PHYRAGO efficacy.
Warnings for Phyrago
Included as part of the "PRECAUTIONS" Section
Precautions for Phyrago
Myelosuppression
Treatment with dasatinib is associated with severe (NCI CTCAE Grade 3 or 4) thrombocytopenia, neutropenia, and anemia, which occur earlier and more frequently in patients with advanced phase CML or Ph+ ALL than in patients with chronic phase CML [see ADVERSE REACTIONS].
In patients with chronic phase CML, perform complete blood counts (CBCs) every 2 weeks for 12 weeks, then every 3 months thereafter, or as clinically indicated. In patients with advanced phase CML or Ph+ ALL, perform CBCs weekly for the first 2 months and then monthly thereafter, or as clinically indicated.
Myelosuppression is generally reversible; withhold, reduce, or discontinue PHYRAGO based on severity [see DOSAGE AND ADMINISTRATION].
Bleeding-Related Events
PHYRAGO can cause serious and fatal bleeding. In all CML or Ph+ ALL clinical studies, Grade ≥3 central nervous system (CNS) hemorrhages, including fatalities, occurred in <1% of patients receiving dasatinib. The incidence of Grade 3/4 hemorrhage occurred in 5.8% of adult patients and generally required treatment interruptions and transfusions. The incidence of Grade 5 hemorrhage occurred in 0.4% of adult patients. The most frequent site of hemorrhage was gastrointestinal [see ADVERSE REACTIONS]. Most bleeding events in clinical studies were associated with severe thrombocytopenia. In addition to causing thrombocytopenia in human subjects, dasatinib caused platelet dysfunction in vitro.
Concomitant medications that inhibit platelet function or anticoagulants may increase the risk of hemorrhage.
Fluid Retention
PHYRAGO may cause fluid retention [see ADVERSE REACTIONS]. After 5 years of follow-up in the adult randomized newly diagnosed chronic phase CML study (n=258), Grade 3 or 4 fluid retention was reported in 5% of patients, including 3% of patients with Grade 3 or 4 pleural effusion. In adult patients with newly diagnosed or imatinib-resistant or -intolerant chronic phase CML, Grade 3 or 4 fluid retention occurred in 6% of patients treated with dasatinib at the recommended dose (n=548). In adult patients with advanced phase CML or Ph+ ALL treated with dasatinib, the recommended dose (n=304), Grade 3 or 4 fluid retention was reported in 8% of patients, including Grade 3 or 4 pleural effusion reported in 7% of patients.
Evaluate patients who develop symptoms of pleural effusion or other fluid retention, such as new or worsened dyspnea on exertion or at rest, pleuritic chest pain, or dry cough, promptly with a chest x-ray or additional diagnostic imaging as appropriate. Fluid retention events were typically managed by supportive care measures that may include diuretics or short courses of steroids. Severe pleural effusion may require thoracentesis and oxygen therapy. Consider dose reduction or treatment interruption [see DOSAGE AND ADMINISTRATION].
Cardiovascular Toxicity
PHYRAGO can cause cardiac dysfunction [see ADVERSE REACTIONS]. After 5 years of follow-up in the randomized newly diagnosed chronic phase CML trial in adults (n=258), the following cardiac adverse reactions occurred: cardiac ischemic events (3.9% dasatinib vs 1.6% imatinib), cardiac-related fluid retention (8.5% dasatinib vs 3.9% imatinib), and conduction system abnormalities, most commonly arrhythmia and palpitations (7.0% dasatinib vs 5.0% imatinib). Two cases (0.8%) of peripheral arterial occlusive disease occurred with imatinib and 2 (0.8%) transient ischemic attacks occurred with dasatinib. Monitor patients for signs or symptoms consistent with cardiac dysfunction and treat appropriately.
Pulmonary Arterial Hypertension
PHYRAGO may increase the risk of developing pulmonary arterial hypertension (PAH) in adult and pediatric patients which may occur any time after initiation, including after more than 1 year of treatment. Manifestations include dyspnea, fatigue, hypoxia, and fluid retention [see ADVERSE REACTIONS]. PAH may be reversible on discontinuation of PHYRAGO. Evaluate patients for signs and symptoms of underlying cardiopulmonary disease prior to initiating PHYRAGO and during treatment. If PAH is confirmed, PHYRAGO should be permanently discontinued.
QT Prolongation
PHYRAGO may increase the risk of prolongation of QTc in patients including those with hypokalemia or hypomagnesemia, patients with congenital long QT syndrome, patients taking antiarrhythmic medicines or other medicinal products that lead to QT prolongation, and cumulative high-dose anthracycline therapy [see ADVERSE REACTIONS]. Correct hypokalemia or hypomagnesemia prior to and during PHYRAGO administration.
Severe Dermatologic Reactions
Cases of severe mucocutaneous dermatologic reactions, including Stevens-Johnson syndrome [see ADVERSE REACTIONS] and erythema multiforme, have been reported in patients treated with dasatinib. Discontinue permanently in patients who experience a severe mucocutaneous reaction during treatment if no other etiology can be identified.
Tumor Lysis Syndrome
Tumor lysis syndrome has been reported in patients with resistance to prior imatinib therapy, primarily in advanced phase disease. Due to potential for tumor lysis syndrome, maintain adequate hydration, correct uric acid levels prior to initiating therapy with PHYRAGO and monitor electrolyte levels. Patients with advanced stage disease and/or high tumor burden may be at increased risk and should be monitored more frequently [see ADVERSE REACTIONS].
Embryo-Fetal Toxicity
Based on limited human data, dasatinib can cause fetal harm when administered to a pregnant woman. Adverse pharmacologic effects of dasatinib, including hydrops fetalis, fetal leukopenia, and fetal thrombocytopenia have been reported with maternal exposure to dasatinib. Advise females of reproductive potential and males with female partners of reproductive potential to use effective contraception during treatment with PHYRAGO and for 30 days after the last dose [see Use In Specific Populations].
Effects On Growth And Development In Pediatric Patients
In pediatric trials of dasatinib in chronic phase CML after at least 2 years of treatment, adverse reactions associated with bone growth and development were reported in 5 (5.2%) patients, one of which was severe in intensity (Growth Retardation Grade 3). These 5 cases included cases of epiphyses delayed fusion, osteopenia, growth retardation, and gynecomastia [see ADVERSE REACTIONS]. Of these 5 cases, 1 case of osteopenia and 1 case of gynecomastia resolved during treatment.
Monitor bone growth and development in pediatric patients.
Hepatotoxicity
PHYRAGO may cause hepatotoxicity as measured by elevations in bilirubin, aspartate aminotransferase (AST), alanine aminotransferase (ALT), and alkaline phosphatase [see ADVERSE REACTIONS]. Monitor transaminases at baseline and monthly or as clinically indicated during treatment. Reduce dose, withhold, or permanently discontinue PHYRAGO based on severity [see DOSAGE AND ADMINISTRATION]. When dasatinib is administered in combination with chemotherapy, liver toxicity in the form of transaminase elevation and hyperbilirubinemia has been observed. Monitor hepatic function when PHYRAGO is used in combination with chemotherapy.
Pediatric use information is approved for Bristol-Myers Squibb Company’s SPRYCEL (dasatinib) tablets. However, due to Bristol-Myers Squibb Company’s marketing exclusivity rights, the drug product is not labeled with that pediatric information.
Patient Counseling Information
Advise the patient to read the FDA-approved patient labeling (PATIENT INFORMATION).
Myelosuppression
Inform patients of the possibility of developing low blood cell counts. Advise patients to immediately report fever particularly in association with any suggestion of infection [see WARNINGS AND PRECAUTIONS].
Bleeding
Inform patients of the possibility of serious bleeding and to report immediately any signs or symptoms suggestive of hemorrhage (unusual bleeding or easy bruising) [see WARNINGS AND PRECAUTIONS].
Fluid Retention
Inform patients of the possibility of developing fluid retention (swelling, weight gain, dry cough, chest pain on respiration, or shortness of breath) and advise them to seek medical attention promptly if those symptoms arise [see WARNINGS AND PRECAUTIONS].
Cardiovascular Toxicity
Inform patients of the possibility of developing cardiovascular toxicity, including cardiac ischemic events, cardiac-related fluid retention, conduction abnormalities, and TIAs. Advise patients to seek immediate medical attention if symptoms suggestive of cardiovascular toxicity occur, such as chest pain, shortness of breath, palpitations, transient vision problems, or slurred speech [see WARNINGS AND PRECAUTIONS].
Pulmonary Arterial Hypertension
Inform patients of the possibility of developing pulmonary arterial hypertension (dyspnea, fatigue, hypoxia, and fluid retention) and advise them to seek medical attention promptly if those symptoms arise [see WARNINGS AND PRECAUTIONS].
Tumor Lysis Syndrome
Inform patients to immediately report and seek medical attention for any symptoms such as nausea, vomiting, weakness, edema, shortness of breath, muscle cramps, and seizures, which may indicate tumor lysis syndrome [see WARNINGS AND PRECAUTIONS].
Growth And Development In Pediatric Patients
Inform pediatric patients and their caregivers of the possibility of developing bone growth abnormalities, bone pain, or gynecomastia and advise them to seek medical attention promptly if those symptoms arise [see WARNINGS AND PRECAUTIONS].
Embryo-Fetal Toxicity
- Advise pregnant women of the potential risk to a fetus [see WARNINGS AND PRECAUTIONS and Use In Specific Populations].
- Advise females of reproductive potential and males with female partners of reproductive potential to use effective contraception during treatment with PHYRAGO and for 30 days after the last dose. Advise females to contact their healthcare provider if they become pregnant, or if pregnancy is suspected, while taking PHYRAGO [see WARNINGS AND PRECAUTIONS and Use In Specific Populations].
Lactation
- Advise women not to breastfeed during treatment with PHYRAGO and for 2 weeks after the last dose [see Use In Specific Populations].
Gastrointestinal Complaints
Inform patients that they may experience nausea, vomiting, or diarrhea with PHYRAGO. Advise patients to seek medical attention if these symptoms are bothersome or persistent.
Advise patients using antacids to avoid taking PHYRAGO and antacids less than 2 hours apart [see DRUG INTERACTIONS].
Pain
Inform patients that they may experience headache or musculoskeletal pain with PHYRAGO. Advise patients to seek medical attention if these symptoms are bothersome or persistent.
Fatigue
Inform patients that they may experience fatigue with PHYRAGO. Advise patients to seek medical attention if this symptom is bothersome or persistent.
Rash
Inform patients that they may experience skin rash with PHYRAGO. Advise patients to seek medical attention if this symptom is bothersome or persistent.
Lactose
PHYRAGO does not contain lactose.
Hepatotoxicity
Advise patients that PHYRAGO can cause hepatotoxicity and that patients with previous history of liver diseases may be at risk. Advise patients to seek immediate medical attention if any symptoms suggestive of hepatotoxicity occur, such as abdominal pain, jaundice and scleral icterus, anorexia, bleeding, bruising, and dark-colored urine [see WARNINGS AND PRECAUTIONS].
Instructions For Taking
PHYRAGO
- Missed Dose
Advise patients that if they miss a dose of PHYRAGO they should take the next scheduled dose at its regular time. The patient should not take two doses at the same time.
- Grapefruit Juice
Advise patients not to drink grapefruit juice as it may increase the amount of dasatinib in their blood and therefore increase their risk of adverse reactions.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment Of Fertility
In a 2-year carcinogenicity study, rats were administered oral doses of dasatinib at 0.3, 1, and 3 mg/kg/day. The highest dose resulted in a plasma drug exposure (AUC) level approximately 60% of the human exposure at 100 mg once daily. Dasatinib induced a statistically significant increase in the combined incidence of squamous cell carcinomas and papillomas in the uterus and cervix of high-dose females and prostate adenoma in low-dose males.
Dasatinib was clastogenic when tested in vitro in Chinese hamster ovary cells, with and without metabolic activation. Dasatinib was not mutagenic when tested in an in vitro bacterial cell assay (Ames test) and was not genotoxic in an in vivo rat micronucleus study.
Dasatinib did not affect mating or fertility in male and female rats at plasma drug exposure (AUC) similar to the human exposure at 100 mg daily. In repeat dose studies, administration of dasatinib resulted in reduced size and secretion of seminal vesicles, and immature prostate, seminal vesicle, and testis. The administration of dasatinib resulted in uterine inflammation and mineralization in monkeys, and cystic ovaries and ovarian hypertrophy in rodents.
Use In Specific Populations
Pregnancy
Risk Summary
Based on limited human data, PHYRAGO can cause fetal harm when administered to a pregnant woman. Adverse pharmacologic effects including hydrops fetalis, fetal leukopenia, and fetal thrombocytopenia have been reported with maternal exposure to dasatinib. Animal reproduction studies in rats have demonstrated extensive mortality during organogenesis, the fetal period, and in neonates. Skeletal malformations were observed in a limited number of surviving rat and rabbit conceptuses. These findings occurred at dasatinib plasma concentrations below those in humans receiving therapeutic doses of dasatinib [see Data]. Advise a pregnant woman of the potential risk to a fetus.
The estimated background risk in the U.S. general population of major birth defects is 2% to 4% and of miscarriage is 15% to 20% of clinically recognized pregnancies.
Clinical Considerations
Fetal/Neonatal Adverse Reactions
Transplacental transfer of dasatinib has been reported. Dasatinib has been measured in fetal plasma and amniotic fluid at concentrations comparable to those in maternal plasma. Hydrops fetalis, fetal leukopenia, and fetal thrombocytopenia have been reported with maternal exposure to dasatinib. These adverse pharmacologic effects on the fetus are similar to adverse reactions observed in adult patients and may result in fetal harm or neonatal death [see WARNINGS AND PRECAUTIONS].
Data
Human Data
Based on human experience, dasatinib is suspected to cause congenital malformations, including neural tube defects, and harmful pharmacological effects on the fetus when administered during pregnancy.
Animal Data
In nonclinical studies at plasma concentrations below those observed in humans receiving therapeutic doses of dasatinib, embryo-fetal toxicities were observed in rats and rabbits. Fetal death was observed in rats. In both rats and rabbits, the lowest doses of dasatinib tested (rat: 2.5 mg/kg/day [15 mg/m2/day] and rabbit: 0.5 mg/kg/day [6 mg/m2/day]) resulted in embryo-fetal toxicities. These doses produced maternal AUCs of 105 ng•h/mL and 44 ng•h/mL (0.1-fold the human AUC) in rats and rabbits, respectively. Embryo-fetal toxicities included skeletal malformations at multiple sites (scapula, humerus, femur, radius, ribs, and clavicle), reduced ossification (sternum; thoracic, lumbar, and sacral vertebrae; forepaw phalanges; pelvis; and hyoid body), edema, and microhepatia. In a pre- and postnatal development study in rats, administration of dasatinib from gestation day (GD) 16 through lactation day (LD) 20, GD 21 through LD 20, or LD 4 through LD 20 resulted in extensive pup mortality at maternal exposures that were below the exposures in patients treated with dasatinib at the recommended labeling dose.
Lactation
Risk Summary
No data are available regarding the presence of dasatinib in human milk, the effects of the drug on the breastfed child, or the effects of the drug on milk production. However, dasatinib is present in the milk of lactating rats. Because of the potential for serious adverse reactions in nursing children from dasatinib, breastfeeding is not recommended during treatment with PHYRAGO and for 2 weeks after the last dose.
Females And Males Of Reproductive Potential
PHYRAGO can cause fetal harm when administered to a pregnant woman [see Pregnancy].
Contraception
Advise females of reproductive potential and males with female partners of reproductive potential to use effective contraception during treatment with PHYRAGO and for 30 days after the last dose.
Infertility
Based on animal data, PHYRAGO may result in damage to female and male reproductive tissues [see Nonclinical Toxicology].
Pediatric Use
Monitor bone growth and development in pediatric patients [see WARNINGS AND PRECAUTIONS].
Pediatric use information is approved for Bristol-Myers Squibb Company’s SPRYCEL (dasatinib) tablets. However, due to Bristol-Myers Squibb Company’s marketing exclusivity rights, this drug product is not labeled with that pediatric information.
Geriatric Use
Of the 2712 patients in clinical studies of dasatinib, 617 (23%) were 65 years of age and older, and 123 (5%) were 75 years of age and older. No differences in confirmed Complete Cytogenetic Response (cCCyR) and MMR were observed between older and younger patients. While the safety profile of dasatinib in the geriatric population was similar to that in the younger population, patients aged 65 years and older are more likely to experience the commonly reported adverse reactions of fatigue, pleural effusion, diarrhea, dyspnea, cough, lower gastrointestinal hemorrhage, and appetite disturbance, and more likely to experience the less frequently reported adverse reactions of abdominal distention, dizziness, pericardial effusion, congestive heart failure, hypertension, pulmonary edema, and weight decrease, and should be monitored closely.
Overdose Information for Phyrago
Experience with overdose of dasatinib in clinical studies is limited to isolated cases. The highest overdosage of 280 mg per day for 1 week was reported in two patients and both developed severe myelosuppression and bleeding. Since dasatinib is associated with severe myelosuppression [see WARNINGS AND PRECAUTIONS and ADVERSE REACTIONS], monitor patients who ingest more than the recommended dosage closely for myelosuppression and give appropriate supportive treatment.
Acute overdose in animals was associated with cardiotoxicity. Evidence of cardiotoxicity included ventricular necrosis and valvular/ventricular/atrial hemorrhage at single doses ≥100 mg/kg (600 mg/m2) in rodents. There was a tendency for increased systolic and diastolic blood pressure in monkeys at single doses ≥10 mg/kg (120 mg/m2).
Contraindications for Phyrago
None.
Clinical Pharmacology for Phyrago
Mechanism Of Action
Dasatinib, at nanomolar concentrations, inhibits the following kinases: BCR-ABL, SRC family (SRC, LCK, YES, FYN), c-KIT, EPHA2, and PDGFRβ. Based on modeling studies, dasatinib is predicted to bind to multiple conformations of the ABL kinase.
In vitro, dasatinib was active in leukemic cell lines representing variants of imatinib mesylatesensitive and resistant disease. Dasatinib inhibited the growth of chronic myeloid leukemia (CML) and acute lymphoblastic leukemia (ALL) cell lines overexpressing BCR-ABL. Under the conditions of the assays, dasatinib could overcome imatinib resistance resulting from BCR-ABL kinase domain mutations, activation of alternate signaling pathways involving the SRC family kinases (LYN, HCK), and multi-drug resistance gene overexpression.
Pharmacodynamics
Cardiac Electrophysiology
Of 2440 patients treated with dasatinib at all doses tested in clinical trials, 16 patients (<1%) had QTc prolongation reported as an adverse reaction. Twenty-two patients (1%) experienced a QTcF > 500 ms. In 865 patients with leukemia treated with dasatinib 70 mg BID in five Phase 2 studies, the maximum mean changes in QTcF (90% upper bound CI) from baseline ranged from 7 ms to 13.4 ms.
An analysis of the data from five Phase 2 studies in patients (70 mg BID) and a Phase 1 study in healthy subjects (100 mg single dose) suggests that there is a maximum increase of 3 to 6 milliseconds in Fridericia corrected QTc interval from baseline for subjects receiving therapeutic doses of dasatinib, with associated upper 95% confidence intervals <10 msec.
Pharmacokinetics
The pharmacokinetics of dasatinib exhibits dose proportional increases in AUC and linear elimination characteristics over the dose range of 15 mg/day (0.15 times the lowest approved recommended dose) to 240 mg/day (1.7 times the highest approved recommended dose).
At 100 mg QD, the maximum concentration at steady state (Cmax) is 82.2 ng/mL (CV% 69%), area under the plasma drug concentration time curve (AUC) is 397 ng/mL*hr (CV% 55%). The clearance of dasatinib is found to be time-invariant.
Absorption
The maximum plasma concentration (Cmax) of dasatinib was observed at a median Tmax (range) of 1.25 hours (0.5 – 3.5 hours) following administration of a single oral 100 mg dose of PHYRAGO.
Effect of Food
No clinically significant differences in dasatinib pharmacokinetics were observed following PHYRAGO administration with a high-fat meal (approximately 900 calories, 50% fat).
Distribution
The geometric mean (%CV) apparent volume of distribution for PHYRAGO is 1850 L (59.3%).
Binding of dasatinib to human plasma proteins in vitro was approximately 96% and of its active metabolite was 93%, with no concentration dependence over the range of 100 ng/mL to 500 ng/mL.
Dasatinib is a P-gp substrate in vitro.
Elimination
The mean terminal half-life of PHYRAGO is 5 hours and the geometric mean (%CV) apparent clearance is 241 L/hr (54.4%).
Metabolism
Dasatinib is metabolized in humans, primarily by CYP3A4. CYP3A4 is the primary enzyme responsible for the formation of the active metabolite. Flavin-containing monooxygenase 3 (FMO-3) and uridine diphosphate-glucuronosyltransferase (UGT) enzymes are also involved in the formation of dasatinib metabolites.
The exposure of the active metabolite, which is equipotent to dasatinib, represents approximately 5% of the AUC of dasatinib. The active metabolite of dasatinib is unlikely to play a major role in the observed pharmacology of the drug. Dasatinib also has several other inactive oxidative metabolites.
Excretion
Elimination is primarily via the feces. Following a single radiolabeled dose of oral dasatinib, 4% of the administered radioactivity was recovered in the urine and 85% in the feces within 10 days. Unchanged dasatinib accounted for 0.1% of the administered dose in the urine and 19% of the administered dose in the feces with the remainder of the dose being metabolites.
Specific Populations
Age, sex, and renal impairment (creatinine clearance 21.6 mL/min to 342.3 mL/min as estimated by Cockcroft Gault) have no clinically relevant effect on the pharmacokinetics of dasatinib.
Pediatric Patients
Pediatric use information is approved for Bristol Myers Squibb Company’s SPRYCEL (dasatinib) tablets. However, due to Bristol Myers Squibb Company’s marketing exclusivity rights, this drug product is not labeled with that pediatric information.
Patients with Hepatic Impairment
Compared to subjects with normal liver function, patients with moderate hepatic impairment (Child Pugh B) had decreases in mean Cmax by 47% and mean AUC by 8%. Patients with severe hepatic impairment (Child Pugh C) had decreases in mean Cmax by 43% and in mean AUC by 28% compared to the subjects with normal liver function.
Drug Interaction Studies
Cytochrome P450 Enzymes
The coadministration of ketoconazole (strong CYP3A4 inhibitor) twice daily increased the mean Cmax of dasatinib by 4-fold and the mean AUC of dasatinib by 5-fold following a single oral dose of 20 mg.
The coadministration of rifampin (strong CYP3A4 inducer) once daily decreased the mean Cmax of dasatinib by 81% and the mean AUC of dasatinib by 82%.
Dasatinib is a time-dependent inhibitor of CYP3A4. Dasatinib does not inhibit CYP1A2, 2A6, 2B6, 2C8, 2C9, 2C19, 2D6, or 2E1. Dasatinib does not induce CYP enzymes.
Gastric Acid Reducing Agents
The dasatinib Cmax decreased by 47.9% and AUC by 31.7% following concomitant use with a calcium carbonate antacid.
No clinically significant differences in the pharmacokinetics of PHYRAGO were observed following concomitant use with omeprazole (proton pump inhibitor) or famotidine (H2 receptor antagonist).
Transporters
Dasatinib is not an inhibitor of P-gp in vitro.
Clinical Studies
Newly Diagnosed Chronic Phase CML In Adults
DASISION (Dasatinib vs Imatinib Study in Treatment-Naive Chronic Myeloid Leukemia Patients) (NCT00481247) was an open-label, multicenter, international, randomized trial conducted in adult patients with newly diagnosed chronic phase CML. A total of 519 patients were randomized to receive either dasatinib 100 mg once daily or imatinib 400 mg once daily. Patients with a history of cardiac disease were included in this trial except those who had a myocardial infarction within 6 months, congestive heart failure within 3 months, significant arrhythmias, or QTc prolongation. The primary endpoint was the rate of confirmed complete cytogenetic response (CCyR) within 12 months. Confirmed CCyR was defined as a CCyR noted on two consecutive occasions (at least 28 days apart).
Median age was 46 years in the dasatinib group and 49 years in the imatinib groups, with 10% and 11% of patients ≥65 years of age, respectively. There were slightly more male than female patients in both groups (59% vs 41%). Fifty-three percent of all patients were Caucasian and 39% were Asian. At baseline, the distribution of Hasford scores was similar in the dasatinib and imatinib treatment groups (low risk: 33% and 34%; intermediate risk: 48% and 47%; high risk: 19% and 19%, respectively). With a minimum of 12 months follow-up, 85% of patients randomized to dasatinib and 81% of patients randomized to imatinib were still on study.
With a minimum of 24 months follow-up, 77% of patients randomized to dasatinib and 75% of patients randomized to imatinib were still on study and with a minimum of 60 months follow-up, 61% and 62% of patients, respectively, were still on treatment at the time of study closure.
Efficacy results are summarized in Table 9.
Table 9: Efficacy Results in a Randomized Newly Diagnosed Chronic Phase CML Trial
| Dasatinib (n=259) |
Imatinib (n=260) |
||
| Confirmed CCyRa Within12months(95%CI) |
76.8% (71.2–81.8) | 66.2% (60.1–71.9) | |
| P-value | 0.007* | ||
| Major Molecular Responseb | |||
| 12 months (95% CI) | 52.1% (45.9–58.3) | 33.8% (28.1–39.9) | |
| P-value | <0.0001 | ||
| 60 months (95% CI) | 76.4% (70.8–81.5) | 64.2% (58.1–70.1) | |
| a Confirmed CCyR is defined as a CCyR noted on two consecutive occasions at least 28 days apart. b Major molecular response (at any time) was defined as BCR-ABL ratios ≤0.1% by RQ-PCR in peripheral blood samples standardized on the International scale. These are cumulative rates representing minimum follow up for the time frame specified. * Adjusted for Hasford score and indicated statistical significance at a pre-defined nominal level of significance. CI = confidence interval. |
|||
After 60 months follow-up, median time to confirmed CCyR was 3.1 months in 215 dasatinib responders and 5.8 months in 204 imatinib responders. Median time to MMR after 60 months follow-up was 9.3 months in 198 dasatinib responders and 15.0 months in 167 imatinib responders.
At 60 months, 8 patients (3%) on the dasatinib arm progressed to either accelerated phase or blast crisis while 15 patients (6%) on the imatinib arm progressed to either accelerated phase or blast crisis.
The estimated 60-month survival rates for dasatinib-and imatinib-treated patients were 90.9% (CI:86.6%–93.8%) and 89.6% (CI: 85.2%–92.8%), respectively. Based on data 5 years after the last patient was enrolled in the trial, 83% and 77% of patients were known to be alive in the dasatinib and imatinib treatment groups, respectively, 10% were known to have died in both treatment groups, and 7% and 13% had unknown survival status in the dasatinib and imatinib treatment groups, respectively.
At 60 months follow-up in the dasatinib arm, the rate of MMR at any time in each risk group determined by Hasford score was 90% (low risk), 71% (intermediate risk) and 67% (high risk). In the imatinib arm, the rate of MMR at any time in each risk group determined by Hasford score was 69% (low risk), 65% (intermediate risk), and 54% (high risk).
BCR-ABL sequencing was performed on blood samples from patients in the newly diagnosed trial who discontinued dasatinib or imatinib therapy. Among dasatinib-treated patients the mutations detected were T315I, F317I/L, and V299L.
Dasatinib does not appear to be active against the T315I mutation, based on in vitro data.
Imatinib-Resistant Or -Intolerant CML Or Ph+ ALL In Adults
The efficacy and safety of dasatinib were investigated in adult patients with CML or Ph+ ALL whose disease was resistant to or who were intolerant to imatinib: 1158 patients had chronic phase CML, 858 patients had accelerated phase, myeloid blast phase, or lymphoid blast phase CML, and 130 patients had Ph+ ALL. In a clinical trial in chronic phase CML, resistance to imatinib was defined as failure to achieve a complete hematologic response (CHR; after 3 months), major cytogenetic response (MCyR; after 6 months), or complete cytogenetic response (CCyR; after 12 months); or loss of a previous molecular response (with concurrent ≥10% increase in Ph+ metaphases), cytogenetic response, or hematologic response. Imatinib intolerance was defined as inability to tolerate 400 mg or more of imatinib per day or discontinuation of imatinib because of toxicity.
Results described below are based on a minimum of 2 years follow-up after the start of dasatinib therapy in patients with a median time from initial diagnosis of approximately 5 years. Across all studies, 48% of patients were women, 81% were white, 15% were black or Asian, 25% were 65 years of age or older, and 5% were 75 years of age or older. Most patients had long disease histories with extensive prior treatment, including imatinib, cytotoxic chemotherapy, interferon, and stem cell transplant. Overall, 80% of patients had imatinib-resistant disease and 20% of patients were intolerant to imatinib. The maximum imatinib dose had been 400–600 mg/day in about 60% of the patients and >600 mg/day in 40% of the patients.
The primary efficacy endpoint in chronic phase CML was MCyR, defined as elimination (CCyR) or substantial diminution (by at least 65%, partial cytogenetic response) of Ph+ hematopoietic cells. The primary efficacy endpoint in accelerated phase, myeloid blast phase, lymphoid blast phase CML, and Ph+ ALL was major hematologic response (MaHR), defined as either a CHR or no evidence of leukemia (NEL).
Chronic Phase CML
Dose-Optimization Trial
A randomized, open-label trial (NCT00123474) was conducted in adult patients with chronic phase CML to evaluate the efficacy and safety of dasatinib administered once daily compared with dasatinib administered twice daily. Patients with significant cardiac diseases, including myocardial infarction within 6 months, congestive heart failure within 3 months, significant arrhythmias, or QTc prolongation were excluded from the trial. The primary efficacy endpoint was MCyR in patients with imatinib-resistant CML. A total of 670 patients, of whom 497 had imatinib-resistant disease, were randomized to the dasatinib 100 mg once-daily, 140 mg once-daily, 50 mg twice-daily, or 70 mg twice-daily group. Median duration of treatment was 22 months.
Efficacy was achieved across all dasatinib treatment groups with the once-daily schedule demonstrating comparable efficacy (non-inferiority) to the twice-daily schedule on the primary efficacy endpoint (difference in MCyR 1.9%; 95% CI [−6.8%–10.6%]); however, the 100-mg once-daily regimen demonstrated improved safety and tolerability.
Efficacy results are presented in Tables 10 and 11 for adult patients with chronic phase CML who received the recommended starting dose of 100 mg once daily.
Table 10: Efficacy of Dasatinib in Adult Patients with Imatinib-resistant or Intolerant Chronic Phase CML (minimum of 24 months follow-up)
| All Patients | 100 mg Once Daily (n=167) |
| Hematologic Response Rate % (95% CI) | |
| CHRa | 92% (86–95) |
| Cytogenetic Response Rate % (95% CI) | |
| MCyRb | 63% (56–71) |
| CCyR | 50% (42–58) |
| a CHR (response confirmed after 4 weeks): WBC ≤ institutional ULN, platelets <450,000/mm3, no blasts or promyelocytes in peripheral blood, <5% myelocytes plus metamyelocytes in peripheral blood, basophils in peripheral blood <20%, and no extramedullary involvement. b MCyR combines both complete (0% Ph+ metaphases) and partial (>0%–35%) responses. |
|
Table 11: Long-Term MMR of Dasatinib in the Dose Optimization Trial: Adult Patients with Imatinib-Resistant or -Intolerant Chronic Phase CMLa
| Minimum Follow-up Period | |||
| 2 Years | 5 Years | 7 Years | |
| Major Molecular Responseb % (n/N) | |||
| All Patients Randomized | 34% (57/167) | 43% (71/167) | 44% (73/167) |
| Imatinib-Resistant Patients | 33% (41/124) | 40% (50/124) | 41% (51/124) |
| Imatinib-Intolerant Patients | 37% (16/43) | 49% (21/43) | 51% (22/43) |
| a Results reported in recommended starting dose of 100 mg once daily. b Major molecular response criteria: Defined as BCR-ABL/control transcripts ≤0.1% by RQ-PCR in peripheral blood samples. |
|||
Based on data 7 years after the last patient was enrolled in the trial, 44% were known to be alive, 31% were known to have died, and 25% had an unknown survival status.
By 7 years, transformation to either accelerated or blast phase occurred in nine patients on treatment in the 100 mg once-daily treatment group.
Advanced Phase CML And Ph+ ALL
Dose-Optimization Trial
One randomized open-label trial (NCT00123487) was conducted in patients with advanced phase CML (accelerated phase CML, myeloid blast phase CML, or lymphoid blast phase CML) to evaluate the efficacy and safety of dasatinib administered once daily compared with dasatinib administered twice daily. The primary efficacy endpoint was MaHR. A total of 611 patients were randomized to either the dasatinib 140 mg once-daily or 70 mg twice-daily group. Median duration of treatment was approximately 6 months for both treatment groups. The once-daily schedule demonstrated comparable efficacy (non-inferiority) to the twice-daily schedule on the primary efficacy endpoint; however, the 140-mg once-daily regimen demonstrated improved safety and tolerability.
Response rates for patients in the 140 mg once-daily group are presented in Table 12.
Table 12: Efficacy of Dasatinib in Imatinib-Resistant or -Intolerant Advanced Phase CML and Ph+ ALL (Two-Year Results)
| 140 mg Once Daily | ||||
| Accelerated (n=158) | Myeloid Blast (n=75) | Lymphoid Blast (n=33) | Ph+ ALL (n=40) | |
| MaHRa | 66% | 28% | 42% | 38% |
| (95% CI) | (59–74) | (18–40) | (26–61) | (23–54) |
| CHRa (95% CI) |
47% (40–56) |
17% (10–28) |
21% (9–39) |
33% (19–49) |
| NELa | 19% | 11% | 21% | 5% |
| (95% CI) | (13–26) | (5–20) | (9–39) | (1–17) |
| MCyRb | 39% | 28% | 52% | 70% |
| (95% CI) | (31–47) | (18–40) | (34–69) | (54–83) |
| CCyR | 32% | 17% | 39% | 50% |
| (95% CI) | (25–40) | (10–28) | (23–58) | (34–66) |
| a Hematologic response criteria (all responses confirmed after 4 weeks): Major hematologic response: (MaHR) = complete hematologic response (CHR) + no evidence of leukemia (NEL). CHR: WBC ≤ institutional ULN, ANC ≥1000/mm3, platelets ≥100,000/mm3, no blasts or promyelocytes in peripheral blood, bone marrow blasts ≤5%, <5% myelocytes plus metamyelocytes in peripheral blood, basophils in peripheral blood <20%, and no extramedullary involvement. NEL: same criteria as for CHR but ANC ≥500/mm3 and <1000/mm3, or platelets ≥20,000/mm3 and ≤100,000/mm3. b MCyR combines both complete (0% Ph+ metaphases) and partial (>0%–35%) responses. CI = confidence interval ULN = upper limit of normal range. |
||||
In the dasatinib 140 mg once-daily group, the median time to MaHR was 1.9 months (min-max: 0.7-14.5) for patients with accelerated phase CML, 1.9 months (min-max: 0.9-6.2) for patients with myeloid blast phase CML, and 1.8 months (min-max: 0.9-2.8) for patients with lymphoid blast phase CML.
In patients with myeloid blast phase CML, the median duration of MaHR was 8.1 months (minmax: 2.7-21.1) and 9.0 (min-max: 1.8-23.1) months for the 140 mg once-daily group and the 70 mg twice-daily group, respectively. In patients with lymphoid blast phase CML, the median duration of MaHR was 4.7 months (min-max: 3.0-9.0) and 7.9 months (min-max: 1.6-22.1) for the 140 mg once-daily group and the 70 mg twice-daily group, respectively. In patients with Ph+ ALL who were treated with dasatinib 140 mg once-daily, the median duration of MaHR was 4.6 months (min-max: 1.4-10.2). The medians of progression-free survival for patients with Ph+ ALL treated with dasatinib 140 mg once-daily and 70 mg twice-daily were 4.0 months (minmax: 0.4-11.1) and 3.1 months (min-max: 0.3-20.8), respectively.
Pediatric use information is approved for Bristol-Myers Squibb Company’s SPRYCEL (dasatinib) tablets. However, due to Bristol-Myers Squibb Company’s marketing exclusivity rights, this drug product is not labeled with that pediatric information.
Patient Information for Phyrago
PHYRAGO
(FYE-rah-go)
(dasatinib) tablets
What is PHYRAGO?
PHYRAGO is a prescription medicine used to treat:
- adults with newly diagnosed Philadelphia chromosome-positive (Ph+) chronic myeloid leukemia (CML) in chronic phase.
- adults with Ph+ CML who no longer benefit from, or did not tolerate, other treatment, including imatinib.
- adults with Ph+ acute lymphoblastic leukemia (Ph+ ALL) who no longer benefit from, or did not tolerate, other treatment.
Before taking PHYRAGO, tell your healthcare provider about all of your medical conditions, including if you:
- have problems with your immune system
- have heart problems, including a condition called congenital long QT syndrome
- have low potassium or low magnesium levels in your blood
- liver problems
- are pregnant or plan to become pregnant. PHYRAGO can harm your unborn baby.
Females who can become pregnant:- You should not become pregnant during treatment with PHYRAGO.
- You should use effective birth control (contraception) during treatment and for 30 days after your last dose of PHYRAGO.
- Talk to your healthcare provider right away if you become pregnant or think you may be pregnant during treatment with PHYRAGO.
- You should use effective birth control (contraception) during treatment and for 30 days after your last dose of PHYRAGO.
- Your female partner should call her healthcare provider if she becomes pregnant or thinks she is pregnant during your treatment with PHYRAGO.
- are breastfeeding or plan to breastfeed. It is not known if PHYRAGO passes into your breast milk. You should not breastfeed during treatment and for 2 weeks after your last dose of PHYRAGO.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, antacids, and herbal supplements. If you take an antacid medicine, take it 2 hours before or 2 hours after your dose of PHYRAGO.
How should I take PHYRAGO?
- Take PHYRAGO exactly as your healthcare provider tells you to take it.
- Your healthcare provider may change your dose of PHYRAGO or temporarily stop treatment with PHYRAGO. Do not change your dose or stop taking PHYRAGO without first talking to your healthcare provider.
- Take PHYRAGO 1 time a day.
- Take PHYRAGO with or without food, either in the morning or in the evening.
- Swallow PHYRAGO whole. Do not crush, cut or chew the tablets.
- You should not drink grapefruit juice during treatment with PHYRAGO.
- If you miss a dose of PHYRAGO, take your next scheduled dose at your regular time. Do not take two doses at the same time.
- If you take too much PHYRAGO, call your healthcare provider or go to the nearest hospital emergency room right away.
What are the possible side effects of PHYRAGO?
PHYRAGO may cause serious side effects, including:
- Low blood cell counts. Low blood cell counts are common with dasatinib and can be severe, including low red blood cell counts (anemia), low white blood cell counts (neutropenia), and low platelet counts (thrombocytopenia). Your healthcare provider will do blood tests to check your blood cell counts regularly during your treatment with PHYRAGO. Call your healthcare provider right away if you have a fever or any signs of an infection during treatment with PHYRAGO.
- Bleeding problems.Bleeding problems are common with PHYRAGO. Sometimes these bleeding problems can be serious and lead to death. Call your healthcare provider right away if you have:
- unusual bleeding or bruising of your skin
- bright red or dark tar-like stools
- decreased alertness, headache, or change in speech
- Your body may hold too much fluid (fluid retention). Fluid retention is common with PHYRAGO and can sometimes be severe. In severe cases, fluid may build up in the lining of your lungs, the sac around your heart, or your stomach cavity. Call your healthcare provider right away if you get any of these symptoms during treatment with PHYRAGO:
- swelling all over your body
- weight gain
- shortness of breath, especially if this happens with low levels of physical activity or at rest
- dry cough
- chest pain when taking a deep breath
- Heart and blood vessel (cardiovascular) problems. PHYRAGO may cause heart problems, including an abnormal heart rate, a heart attack, or small strokes that last only a few minutes or a few hours, called transient ischemic attacks (TIAs). TIAs are often a warning sign that you are at risk for a more serious stroke. Your healthcare provider will monitor the potassium and magnesium levels in your blood and your heart function. Get medical help right away if you develop any of the following symptoms during treatment with PHYRAGO:
- chest pain
- shortness of breath
- feeling like your heart is beating too fast or you feel abnormal heart beats
- vision changes that may last for a short time
- slurred speech
- Pulmonary Arterial Hypertension (PAH). PHYRAGO may cause high blood pressure in the vessels of your lungs. PAH may happen at any time during your treatment with PHYRAGO. Your healthcare provider should check your heart and lungs before and during treatment with PHYRAGO. Call your healthcare provider right away if you have shortness of breath, tiredness, or swelling all over your body (fluid retention).
- Severe skin reactions. PHYRAGO may cause skin reactions that can sometimes be severe. Get medical help right away if you get a skin reaction with fever, sore mouth or throat, or blistering or peeling of your skin or in the mouth.
- Tumor Lysis Syndrome (TLS).TLS is caused by a fast breakdown of cancer cells. TLS can cause you to have kidney failure and the need for dialysis treatment, and an abnormal heartbeat. Your healthcare provider may do blood tests to check you for TLS. Call your healthcare provider or get emergency medical help right away if you develop any of these symptoms during treatment with PHYRAGO:
- Nausea
- shortness of breath
- vomiting
- muscle cramps
- weakness
- seizures
- swelling
- Slowing of growth and development in children.Effects on bone growth and development in children have happened with dasatinib and can sometimes be severe. Your healthcare provider will monitor your child’s bone growth and development during treatment with dasatinib. Get medical help right away if your child develops bone pain.
- Liver problems.PHYRAGO can cause liver problems. People who have had liver problems in the past may be at risk for getting liver problems with PHYRAGO. Your healthcare provider will monitor your liver function as needed during treatment with PHYRAGO. Call your healthcare provider or get medical help right away if you develop any symptoms of liver problems, including:
- stomach-area (abdominal) pain
- bleeding
- yellowing of your skin or white part of your eyes
- bruising
- loss of appetite
- dark “tea-colored” urine
The most common side effects in adults receiving dasatinib alone include:
- diarrhea
- tiredness
- headache
- nausea
- skin rash
- muscle pain
- shortness of breath
PHYRAGO may cause fertility problems in males and females. Talk to your healthcare provider if this is a concern for you.
Tell your healthcare provider if you have any side effect that bothers you or that does not go away. These are not all of the possible side effects of PHYRAGO.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store PHYRAGO?
- Store PHYRAGO at room temperature between 68°F to 77°F (20°C to 25°C).
- PHYRAGO comes in a bottle with a child-resistant closure.
- PHYRAGO bottles contain a desiccant container (drying agent) to help keep the medicine dry. Keep the desiccant container in the PHYRAGO bottle after opening. Do not swallow or eat the desiccant container.
- Ask your healthcare provider or pharmacist about the right way to throw away expired or unused PHYRAGO.
- Wear latex or nitrile gloves when handling PHYRAGO tablets.
- Females who are pregnant should not handle PHYRAGO.
Keep PHYRAGO and all medicines out of the reach of children.
General information about the safe and effective use of PHYRAGO.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use PHYRAGO for a condition for which it is not prescribed. Do not give PHYRAGO to other people even if they have the same symptoms you have. It may harm them. You can ask your healthcare provider or pharmacist for information about PHYRAGO that is written for health professionals.
What are the ingredients in PHYRAGO?
Active ingredient:dasatinib (anhydrous)
Inactive ingredients: croscarmellose sodium, dibasic calcium phosphate, magnesium stearate, methacrylic acid-ethyl acrylate copolymer, microcrystalline cellulose, propyl gallate, and silica dimethyl silylate.
Pediatric use information is approved for Bristol-Myers Squibb Company’s SPRYCEL (dasatinib) tablets. However, due to Bristol-Myers Squibb Company’s marketing exclusivity rights, this drug product is not labeled with that pediatric information.
This Patient Information has been approved by the U.S. Food and Drug Administration.
From 

Report Problems to the Food and Drug Administration
You are encouraged to report negative side effects of prescription drugs to the FDA. Visit the FDA MedWatch website or call 1-800-FDA-1088.