WARNING
RISK OF POTENTIAL ADVERSE EMBOLIC EFFECTS RESULTING FROM INADVERTENT INTRAVASCAULAR INJECTION
- Inadvertent intravascular injection could cause POSIMIR droplets to be deposited in the pulmonary and other capillary beds. Administer POSIMIR into the subacromial space at the end of arthroscopic shoulder surgery. Direct arthroscopic visualization must be used to confirm proper placement of the needle tip before injecting POSIMIR. [see WARNINGS AND PRECAUTIONS].
Description for Posimir
POSIMIR (bupivacaine solution) is a sterile nonpyrogenic, clear, light yellow to amber solution for infiltration. Over time, the solution color will intensify within the range, from light yellow to amber. The range of color is not associated with a change in potency of the drug product. POSIMIR contains bupivacaine (132 mg/mL), benzyl alcohol, and sucrose acetate isobutyrate.
Bupivacaine, an amide-type local anesthetic, is 1-butyl-N-(2,6-dimethylphenyl)-2-piperidinecarboxamide. It is a white crystalline powder with a molecular weight of 288.43 g/mol. The structure of bupivacaine is shown below:
 |
Bupivacaine is present in POSIMIR at a concentration of 132 mg/mL.
Uses for Posimir
POSIMIR is indicated in adults for administration into the subacromial space under direct arthroscopic visualization to produce post-surgical analgesia for up to 72 hours following arthroscopic subacromial decompression.
Limitations Of Use
Safety and effectiveness have not been established in other surgical procedures, including soft tissue surgical procedures, other orthopedic procedures, including for intra-articular administration, and boney procedures, or when used for neuraxial or peripheral nerve blockade.
Dosage for Posimir
Important Dosage And Administration Information
- POSIMIR is intended for single-dose administration only.
- Do not dilute or mix POSIMIR with local anesthetics or other drugs or diluents.
- As there is a potential risk of severe, life-threatening adverse reactions associated with the administration of bupivacaine, POSIMIR should be administered in a setting where trained personnel and equipment are available to promptly treat patients who show evidence of neurological or cardiac toxicity.
- Different formulations of bupivacaine are not bio equivalent to POSIMIR even if the milligram dosage is the same. It is not possible to convert dosing from any other formulations of bupivacaine to POSIMIR and vice versa. Do not substitute.
- The toxic effects of local anesthetics are additive. Avoid additional use of local anesthetics within 168 hours following administration of POSIMIR.
- Avoid intra vascular administration of POSIMIR. Convulsions and cardiac arrest have occurred following accidental intra vascular injection of bupivacaine and other amide-containing products.
- POSIMIR is not indicated for the following routes of administration.
- Epidural
- Intrathecal
- intra vascular
- Intra-articular use [see Nonclinical Toxicology]
- Regional nerve blocks
- Pre-incisional or pre-procedural locoregional anesthetic techniques that require deep and complete sensory block in the area of administration.
Recommended Dose
The recommended dose of POSIMIR is 660 mg (5 mL).
Preparation, Administration, And Dosing Instructions
- POSIMIR is ready to use and does not require dilution or mixing.
- Prior to administration, draw up POSIMIR into a 5 mL syringe using a large bore needle (16 gauge or larger). Once the syringe has been filled, discard the large bore needle.
- At the close of surgery, administer the entire 5 mL dose of POSIMIR into the subacromial space using an 18 gauge or larger-bore needle. The needle may be inserted through an existing arthroscopic port or through intact skin to reach the subacromial space. Confirm correct placement of the needle tip within the subacromial space by direct arthroscopic visualization.
- Do not administer POSIMIR into the glenohumeral intra-articular space.
Compatibility Considerations
POSIMIR is compatible with:
- Commonly implantable materials, such as polypropylene and polyester
- Silk, nylon, gut, polypropylene, polydioxanone, and polyglycolic acid sutures
HOW SUPPLIED
Dosage Forms And Strengths
POSIMIR (bupivacaine solution) is a sterile, nonpyrogenic, clear, light yellow to amber solution in a clear, glass vial.
- 5 mL single-dose vial: 660 mg/5 mL (132 mg/mL)
Storage And Handling
POSIMIR (bupivacaine solution) is available in single-dose vials. It is a sterile nonpyrogenic, clear, light yellow to amber solution in glass vials.
5 mL single-dose vial, 660 mg/5 mL (132 mg/mL) packaged in a 10-unit carton (NDC 51715-660-10 )
Storage
POSIMIR vial should be stored at a controlled room temperature of 20°C to 25°C (68°F to 77°F), excursions permitted to 15°C to 30°C (59°F to 86°F). [See USP Controlled Room Temperature]. Vial should be protected from light and retained in carton until time of use.
Handling
- Do not administer any solution which contains particulate matter.
- Do not autoclave.
- Do not dilute.
- Discard any unused portion in an appropriate manner.
| NDC No. | Container | Size | Quantity |
| NDC 51715-660-10 | Single-dose vials | 5 mL | Box of 10 |
Distributed by Innocoll Pharmaceuticals Limited, Athlone, Ireland N37 VW42. Revised: Feb 2022.
Side Effects for Posimir
The following adverse reactions to bupivacaine hydrochloride are described in other sections of the prescribing information:
- Systemic Toxicity with Intravascular Injection [see WARNINGS AND PRECAUTIONS]
- Methemoglobinemia [see WARNINGS AND PRECAUTIONS]
- Chondrolysis with Intra-Articular Infusion [see WARNINGS AND PRECAUTIONS]
- Cardiovascular System Reactions [see WARNINGS AND PRECAUTIONS]
Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates observed in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety of POSIMIR, doses ranging from 2.5 mL to 5 mL, was evaluated in 10 randomized, double-blind, controlled trials. Overall, POSIMIR 5 mL, the recommended dose, has been administered to a total of 735 patients in clinical trials using a variety of administration methods, including the recommend method of infiltration. Three trials were controlled with bupivacaine HCl, two trials were controlled with bupivacaine HCl and vehicle placebo, and five trials were controlled with vehicle placebo. An additional 47 patients were treated with saline placebo in one of the bupivacaine HCl-controlled trials. The evaluated surgical procedures included inguinal hernia repair, subacromial decompression of the shoulder, abdominal hysterectomy, laparotomy, laparoscopic cholecystectomy, and laparoscopically-assisted colectomy.
Shoulder Surgical Procedures
There were three studies evaluating the safety of POSIMIR administered during shoulder surgery.
In Study 1, one of three treatments was administered into the subacromial space at the end of surgery: POSIMIR, vehicle placebo, or bupivacaine HCl. Table 1 presents commonly-reported adverse reactions from Study 1.
Table 1. Commonly Reported Adverse Reactions from Study 1 (Incidence ≥ 2% and More Frequent than
| Preferred Term, n (%) | Posimir (N=53) |
Bupivacaine HCl (n=29) |
Vehicle Placebo (N=25) |
| Headache | 3 (5.7%) | 1 (3.4%) | 1 (4.0%) |
| Electrocardiogram T wave inversion | 2 (3.8%) | 0 | 0 |
| Hypoesthesia | 2 (3.8%) | 1 (3.4%) | 1 (4.0%) |
| Pruritus generalized | 2 (3.8%) | 0 | 0 |
In Study 2 and Study 3, patients were administered either POSIMIR or vehicle placebo into the subacromial space at the end of surgery. Table 2 presents commonly-reported adverse reactions from Studies 2 and 3.
Table 2. Commonly Reported Adverse Reactions Pooled from Study 2 and Study 3 (Incidence ≥ 2% and
| Preferred Term, n (%)* | Posimir (N=75) |
Vehicle Placebo (N=44) |
| Dizziness | 30 (40.3%) | 17 (38.3%) |
| Vomiting | 22 (29.0%) | 12 (26.6%) |
| Headache | 17 (23.3%) | 7 (16.3%) |
| Paresthesia | 14 (18.4%) | 7 (15.4%) |
| Dysgeusia | 13 (17.3%) | 7 (14.9%) |
| Hypoesthesia | 13 (17.3%) | 7 (15.8%) |
| Tinnitus | 10 (13.2%) | 3 (6.7%) |
| Dysuria | 8 (10.1%) | 4 (10.1%) |
| Pyrexia | 7 (9.3%) | 2 (4.6%) |
| Insomnia | 5 (7.1%) | 0 |
| Dyspnea | 3 (3.8%) | 0 |
| Muscle twitching | 3 (3.8%) | 0 |
| Peripheral swelling | 3 (3.9%) | 0 |
| Urinary retention | 2 (2.7%) | 1 (2.1%) |
| Contusion | 2 (2.5%) | 0 |
| Dysmenorrhea | 2 (2.7%) | 0 |
| Dysmenorrhea | 2 (2.7%) | 0 |
| Nasal congestion | 2 (2.5%) | 0 |
| Pruritus generalized | 2 (2.5%) | 0 |
| * Percentages adjusted to account for the different sizes of the pooled studies. | ||
Less common adverse reactions (incidence less than 2% and more frequent than either bupivacaine HCl or vehicle placebo) following POSIMIR administration in shoulder surgical procedures were angina pectoris, blepharospasm, electrocardiogram T wave amplitude decreased, fatigue, osteoarthritis, procedural nausea, proceduralpain, and pulmonary arterial hypertension.
Additional follow-up safety data consisting of shoulder MRI, physical examination of the shoulder, and assessments of wound healing were collected at 6 months in Study 1 and at 18 months in Study 2. There were no specific long-term follow-up evaluations for patients treated in Study 3; however, study investigators did not report any cases of chondrolysis in follow-up surveys. All surgical incisions were found to have healed as expected in all three studies. Table 3 presents the results of the MRIand physical examinations for Study 1. Table 4 presents the results of the MRI and physical examinations for Study 2.
Table 3. 6-Month Follow-up Safety Data from Subacromial Decompression Study 1.
| Safety Evaluation | Posimir | Vehicle Placebo | Bupivacaine HCl |
| Number enrolled | 53 | 25 | 29 |
| Shoulder MRI | |||
| Number at 6-month follow-up | 51 | 25 | 25 |
| Improved , n (%) | 6 (11.8%) | 2 (8.0%) | 6 (24.0%) |
| No change , n (%) | 31 (60.8%) | 14 (56.0%) | 9 (36.0%) |
| Worsened , n (%) | 14 (27.4%) | 9 (36%) | 10 (40%) |
| Constant-Murley score | |||
| Number at 6-month follow-up | 52 | 25 | 26 |
| Pre-surgery, mean (SD) | 44.7 (12.5%) | 41.7 (11.7%) | 42 (11.3%) |
| Follow-up, mean (SD) | 61.6 (15.2%) | 63.2 (12.4%) | 65.6 (6.8%) |
| Decreased from baseline. n (%) | 5 (9.6%) | 2 (8.0%) | 0 (0%) |
| [1] Compared with pre-surgical MRI | |||
Table 4. 18-Month Follow-up Safety Data from Subacromial Decompression Study 2.
| Safety Evaluation | Posimir | Vehicle Placebo |
| Number enrolled | 40 | 20 |
| Shoulder MRI | ||
| Number at 18-month follow-up | 27 | 14 |
| Overall assessment | ||
| Unexpected injuries or findings compared with pre-surgical MRI, n (%) | 0 | 0 |
| New-onset cartilage or bone lesions of concern (not present at baseline and unrelated to surgery or natural disease progression), n (%) | 0 | 0 |
| Glenohumeral joint and humeral head | ||
| Presence of cartilage thinning - humeral head, n (%) | ||
| Grade 0: normal/none | 26 (96.3%) | 14 (100%) |
| Grade 1: mild | 0 | 0 |
| Grade 2: moderate | 1 (3.7%)[1] | 0 |
| Grade 3: severe | 0 | 0 |
| Rotator cuff and labrum | ||
| Supraspinatus tendon tear, n (%) | ||
| No tear | 16 (59.3%) | 5 (35.7%) |
| Partial | 7 (25.9%) | 7 (50.0%) |
| Full thickness | 1 (3.7%) | 0 |
| Other findings | 3 (11.1%) | 2 (14.3%) |
| Supraspinatus - other findings, n (%) | ||
| Interstitial tear | 0 | 0 |
| Tendinosis | 2 (7.4%) | 1 (7.1%) |
| Surgically repaired tendon | 0 | 1 (7.1%) |
| Interstitial tear/tendinosis | 1 (3.7%) | 0 |
| (blank) | 24 (88.9%) | 12 (85.7%) |
| Subacromial space - acromion | ||
| Acromion bony spur, n (%) | ||
| Yes | 1 (3.7%) | 0 |
| No | 26 (96.3%) | 14 (100%) |
| Acromion bone resection, n (%) | ||
| Yes | 18 (66.7%) | 9 (64.3%) |
| No | 9 (33.3%) | 5 (35.7%) |
| Acromion signal abnormality (edema, fibrosis), n (%) | ||
| Yes | 2 (7.4%)[2] | 2 (14.3%)[2] |
| No | 25 (92.6%)[2] | 12 (85.7%)[2] |
| Acromioclavicular joint | ||
| Bone resection at acromioclavicular joint/postoperative changes, n (%) | ||
| Yes | 10 (37.0%) | 4 (28.6%) |
| No | 16 (59.3%) | 10 (71.4%) |
| Not evaluable | 1 (3.7%) | 0 |
| Joint effusion/synovitis, n (%) | ||
| Grade 0: normal/none | 9 (33.3%) | 2 (14.3%) |
| Grade 1: mild | 9 (33.3%)[3] | 7 (50.0%) |
| Grade 2: moderate | 3 (11.1%)[3] | 3 (21.4%)[3] |
| Grade 3: severe | 0 | 1 (7.1%)[3] |
| Not evaluable | 6 (22.2%) | 1 (7.1%) |
| Bursa and Soft Tissue | ||
| Subacromial bursa - bursitis/excess fluid, n (%) | ||
| Grade 0: normal/none | 18 (66.7%) | 5 (35.7%) |
| Grade 1: mild | 6 (22.2%) | 9 (64.3%)[4] |
| Grade 2: moderate | 3 (11.1%) | 0 |
| Grade 3: severe | 0 | 0 |
| Physical Exam | ||
| Number at 18-month follow-up | 31 | 16 |
| Clinical assessment, n (%) | ||
| Normal | 27 (87.1%) | 13 (81.3%) |
| Abnormal | 4 (12.9%) | 3 (18.8%) |
| Pain intensity, 0-10 scale | ||
| Mean (SE) | 0.9 (0.4%) | 1.2 (0.6%) |
| Positive impingement sign, n (%) | ||
| Yes | 3 (9.7%) | 3 (18.8%) |
| No | 28 (90.3%) | 13 (81.3%) |
| Full passive range of motion, n (%) | ||
| Yes | 27 (87.1%) | 13 (81.3%) |
| No | 4 (12.9%) | 3 (18.8%) |
| [1] The humeral head cartilage thinning was unchanged from baseline. [2] Two POSIMIR patients were positive for signal abnormalities at baseline and negative at 18 months. Two POSIMIR patients were negative for signal abnormalities at baseline and positive at 18 months. One placebo patient was positive for signal abnormality at baseline and negative at 18 months. Two placebo patients were positive for signal abnormalities at both baseline and 18 months. [3] Two POSIMIR patients had joint effusion/synovitis that improved from moderate at baseline to mild at 18 months. One POSIMIR patient had joint effusion/synovitis that improved from severe at baseline to mild at 18 months. Four patients (1 POSIMIR, 3 placebo) had joint effusion/synovitis that worsened from mild at baseline to moderate at 18 months. One placebo patient had joint effusion/synovitis that worsened from mild at baseline to severe at 18 months. [4] One placebo patient had bursitis/excess fluid that was severe at baseline and mild at 18 months. |
||
Soft Tissue Surgical Procedures
There were two studies evaluating the safety of POSIMIR in patients undergoing inguinal hernia repair (hernioplasty). Patients in these studies were administered either POSIMIR 5 mL or vehicle placebo; 2.5 mL administered into the floor of the inguinal canal and 2.5 mL administered into the subcutaneous space. Table 5 presents commonly-reported adverse reactions from these studies.
Table 5. Commonly Reported Adverse Reactions Pooled from Studies in Inguinal Hernia Repair (Incidence ≥ 2% and More Frequent than Placebo)
| Preferred Term, n (%)* | Posimir (N=69) |
Vehicle Placebo (N=53) |
| Bradycardia | 16 (22.9%) | 7 (14.2%) |
| Pruritus† | 15 (21.6%) | 9 (17.5%) |
| Post procedural contusion (bruising) | 10 (14.0%) | 5 (10.1%) |
| Vomiting | 6 (9.4%) | 4 (7.4%) |
| Incision site swelling | 4 (6.0%) | 3 (5.7%) |
| Dyspepsia | 4 (5.7%) | 2 (3.7%) |
| Pyrexia | 4 (6.0%) | 2 (4.0%) |
| Contusion | 4 (5.7%) | 0 |
| Back pain | 3 (4.1%) | 2 (3.4%) |
| Viral infection | 3 (4.1%) | 2 (4.0%) |
| Incision site erythema | 3 (4.1%) | 0 |
| Oropharyngeal pain | 3 (4.6%) | 0 |
| Tachycardia | 3 (4.6%) | 0 |
| Upper respiratory tract infection | 2 (3.0%) | 1 (2.0%) |
| Dry throat | 2 (3.2%) | 0 |
| Hyperhidrosis | 2 (3.0%) | 0 |
| Hypertension | 2 (2.8%) | 0 |
| Local swelling | 2 (3.0%) | 0 |
| Testicular swelling | 2 (3.2%) | 0 |
| * Percentages adjusted to account for the different sizes of the pooled studies. † Incision site pruritus, generalized pruritus, and genital pruritus were also reported, but none had incidence ≥2% and more frequent than placebo. |
||
There were five studies evaluating the safety of POSIMIR in laparoscopic, laparoscopically-assisted, or open abdominal surgeries.
In two studies in patients undergoing laparoscopic cholecystectomy, POSIMIR or bupivacaine HCl was administered into the laparoscopic port incisions at the end of surgery. In one of these studies, a subset of patients received either POSIMIR or saline placebo. In a study of patients undergoing laparoscopically-assisted colectomy, POSIMIR or vehicle placebo was administered predominantly into the hand port incision at the end of surgery. In a study of patients undergoing laparotomy, POSIMIR or bupivacaine HCl was administered into the full length of the surgical incision at the end of surgery. Table 6 presents commonly-reported adverse reactions from these four studies. Table 7 and Table 8 present, respectively, surgical-site adverse reactions and early-occurring central nervous system (CNS)-related adverse reactions from the laparoscopic cholecystectomy study that included a saline placebo control arm.
Table 6. Commonly Reported Adverse Reactions Pooled from Laparoscopic, Laparoscopically-Assisted, and Open Abdominal Surgery Studies (Incidence ≥2% and More Frequent than Bupivacaine HCl or Placebo).
| Preferred Term, n (%)* | Posimir (N=337) |
Bupivacaine HCl (N=186) |
Vehicle Placebo (N=78) |
| Post procedural contusion (bruising) | 231 (71.2%) | 119 (61.8%) | 41 (52.6%) |
| Nausea | 189 (55.8%) | 111 (59.6%) | 40 (51.3%) |
| Constipation | 112 (35.2%) | 80 (41.8%) | 8 (10.3%) |
| Somnolence | 92 (30.4%) | 80 (41.0%) | 3 (3.8%) |
| Headache | 86 (27.2%) | 63 (32.3%) | 12 (15.4%) |
| Dizziness | 75 (23.5%) | 58 (30.1%) | 6 (7.7%) |
| Vomiting | 66 (19.4%) | 39 (21.0%) | 6 (7.7%) |
| Dysgeusia | 50 (16.2%) | 33 (16.9%) | 2 (2.6%) |
| Pruritus† | 45 (14.3%) | 36 (18.7%) | 5 (6.4%) |
| Procedural pain | 35 (11.4%) | 35 (17.8%) | 0 |
| Diarrhea | 34 (9.8%) | 10 (5.5%) | 10 (12.8%) |
| Incision site hemorrhage | 30 (8.7%) | 6 (3.0%) | 3 (3.8%) |
| Pyrexia | 29 (8.2%) | 10 (5.7%) | 11 (14.1%) |
| Abdominal distension | 29 (8.2%) | 8 (4.8%) | 12 (15.4%) |
| Incision site erythema | 29 (8.1%) | 5 (2.7%) | 10 (12.8%) |
| Post procedural discharge | 26 (7.5%) | 8 (4.4%) | 7 (9.0%) |
| Paresthesia | 22 (5.9%) | 25 (13.1%) | 2 (2.6%) |
| Hypokalemia | 18 (5.2%) | 2 (1.4%) | 10 (12.8%) |
| Incision site hematoma | 17 (4.5%) | 3 (1.7%) | 4 (5.1%) |
| Anemia | 16 (4.6%) | 1 (0.7%) | 7 (9.0%) |
| Flatulence | 16 (4.6%) | 7 (4.0%) | 8 (10.3%) |
| Hypertension | 16 (4.5%) | 7 (3.6%) | 1 (1.3%) |
| Incision site infection | 15 (4.2%) | 4 (2.5%) | 2 (2.6%) |
| Musculoskeletal pain | 15 (4.4%) | 8 (4.9%) | 0 |
| Abdominal pain | 14 (3.9%) | 1 (0.5%) | 1 (1.3%) |
| Insomnia | 13 (3.8%) | 5 (2.9%) | 7 (9.0%) |
| Dyspepsia | 13 (3.6%) | 3 (1.9%) | 4 (5.1%) |
| Wound dehiscence | 13 (3.6%) | 3 (1.5%) | 5 (6.4%) |
| Cough | 12 (3.6%) | 3 (1.8%) | 1 (1.3%) |
| Oropharyngeal pain | 12 (3.5%) | 2 (1.0%) | 0 |
| Urinary retention | 10 (2.8%) | 2 (1.2%) | 4 (5.1%) |
| Chest pain | 10 (2.9%) | 1 (0.5%) | 1 (1.3%) |
| Ileus | 10 (2.7%) | 1 (0.7%) | 3 (3.8%) |
| Body temperature increased | 9 (2.4%) | 0 | 2 (2.6%) |
| Abdominal pain upper | 8 (2.5%) | 2 (1.0%) | 0 |
| Rash | 7 (2.1%) | 7 (3.8%) | 1 (1.3%) |
| Pain in extremity | 7 (2.1%) | 5 (2.8%) | 1 (1.3%) |
| Dry mouth | 7 (2.2%) | 2 (1.0%) | 1 (1.3%) |
| Nasopharyngitis | 7 (2.1%) | 0 | 0 |
| * Percentages adjusted to account for the different sizes of the pooled studies. † Incision site pruritus, generalized pruritus, eye pruritus, anal pruritus, and infusion site pruritus were also reported, but none had incidence ≥2% and more frequent than placebo. |
|||
Table 7. Incidence of Surgical Site Adverse Reactions from Laparoscopic Cholecystectomy Study with Saline Placebo and Bupivacaine HCl Controls.
| Prespecified Term*, n (%) | Saline Placebo Control | Bupivacaine HCl Control | ||
| Posimir (N=45) |
Saline Placebo (N=47) |
Posimir (N=148) |
Bupivacaine HCl (N=148) |
|
| Visible bruising | 41 (91.1%) | 33 (70.2%) | 142 (95.9%) | 105 (70.9%) |
| Surgical site bleeding | 22 (48.9%) | 20 (42.6%) | 19 (12.8%) | 24 (16.2%) |
| Drainage from surgical incision(s) | 2 (4.4%) | 3 (6.4%) | 11 (7.4%) | 6 (4.1%) |
| Wound hematoma | 0 | 0 | 6 (4.1%) | 2 (1.4%) |
| Wound dehiscence | 0 | 0 | 2 (1.4%) | 3 (2.0%) |
| Surgical site infection | 0 | 0 | 2 (1.4%) | 1 (0.7%) |
| * Terms were prespecified for examination by a blinded assessor on postoperative days 0, 4, 7, 14, 28, and 59. | ||||
Table 8. CNS-related Adverse Reactions Solicited from Subjects at 6 Hours Post-surgery in Laparoscopic Cholecystectomy Study with Saline Placebo andBupivacaine HCl Controls.
| Dictionary-Derived Term (Symptom)* | Saline Placebo Control | Bupivacaine HCl Control | ||
| Posimir (N=45) |
Saline Placebo (N=47) |
Posimir (N=148) |
Bupivacaine HCl (N=148) |
|
| Entire study, n (%) | ||||
| Somnolence (Drowsiness) | 18 (40.0%) | 16 (34.0%) | 60 (40.5%) | 48 (32.4%) |
| Nausea (Nausea) | 9 (20.0%) | 13 (27.7%) | 48 (32.4%) | 57 (38.5%) |
| Dizziness (Dizziness) | 3 (6.7%) | 3 (6.4%) | 28 (18.9%) | 31 (20.9%) |
| Headache (Headache) | 5 (11.1%) | 4 (8.5%) | 23 (15.5%) | 18 (12.2%) |
| Vomiting (Vomiting) | 2 (4.4%) | 3 (6.4%) | 10 (6.8%) | 15 (10.1%) |
| Constipation (Constipation) | 0 (0.0%) | 4 (8.5%) | 9 (6.1%) | 10 (6.8%) |
| Pruritus (Itching) | 1 (2.2%) | 1 (2.1%) | 6 (4.1%) | 5 (3.4%) |
| Subset of study, n (%)N=23N=22 | ||||
| Dysgeusia (Metallic taste in mouth) | 3 (13.0%) | 2 (9.1%) | 26 (17.6%) | 22 (14.9%) |
| Paresthesia (Tingling) | 0 | 0 | 2 (1.4%) | 6 (4.1%) |
| Hypoesthesia (Numbness) | 0 | 0 | 1 (0.7%) | 1 (0.7%) |
| * Patients responded to a 10-symptom checklist (7 symptoms for part of the saline placebo-controlled portion of the study). | ||||
In a study of patients undergoing total abdominal hysterectomy, POSIMIR, vehicle placebo, or bupivacaine HCl was administered into the surgical incision at the end of surgery. Table 9 presents commonly-reported adverse reactions from this study.
Table 9. Commonly Reported Adverse Reactions from Total Abdominal Hysterectomy Study (Incidence ≥ 2% and More Frequent than Bupivacaine HCl).
| Preferred Term | Posimir (N=60) |
Bupivacaine HCl (N=27) |
Vehicle Placebo (N=27) |
| Post procedural contusion (bruising) | 36 (60.0%) | 0 | 9 (33.3%) |
| Anemia | 10 (16.7%) | 3 (11.1%) | 4 (14.8%) |
| Dizziness | 9 (15.0%) | 4 (14.8%) | 3 (11.1%) |
| Vomiting | 9 (15.0%) | 4 (14.8%) | 8 (29.6%) |
| C-reactive protein increased | 7 (11.7%) | 1 (3.7%) | 0 |
| Pyrexia | 7 (11.7%) | 7 (25.9%) | 3 (11.1%) |
| Somnolence | 5 (8.3%) | 2 (7.4%) | 0 |
| Blood potassium decreased | 4 (6.7%) | 0 | 1 (3.7%) |
| Hypertension | 4 (6.7%) | 2 (7.4%) | 1 (3.7%) |
| Incision site hematoma | 3 (5.0%) | 0 | 0 |
| Electrocardiogram change | 2 (3.3%) | 0 | 0 |
| Procedural hemorrhage | 2 (3.3%) | 0 | 0 |
| Vaginal hematoma | 2 (3.3%) | 0 | 0 |
Less common adverse reactions (incidence less than 2% and more frequent than either bupivacaine HCl or placebo) following POSIMIR administration in soft tissue surgical procedures were: application site irritation, atrial fibrillation, drug eruption, electrocardiogram QT prolonged, eructation, erythema, excessive granulation tissue, fatigue, genital pain, heart rate increased, hiccups, hypoesthesia, hypogeusia, incision site cellulitis, incision site erosion, incision site hypoesthesia, incision siteinflammation, incision site edema, incision site pain, incision site rash, mean arterial pressure increased, micturition urgency, night sweats, overdose, palpitations, procedural hypertension, pruritus generalized, rash generalized, seroma, sinus tachycardia, skin discoloration, tinnitus, and wound hemorrhage.
Drug Interactions for Posimir
Do not dilute or mix POSIMIR with local anesthetics or other drugs or diluents.
Avoid additional use of local anesthetics within 168 hours following administration of POSIMIR.
Drugs Associated With Methemoglobinemia
Patients who are administered POSIMIR are at increased risk of developing methemoglobinemia when concurrently exposed to following drugs, which could include other local anesthetics [see WARNINGS AND PRECAUTIONS].
Examples of Drugs Associated with Methemoglobinemia:
| Class | Examples |
| Nitrates/Nitrites | nitric oxide, nitroglycerin, nitroprusside, nitrous oxide |
| Local anesthetics | articaine, benzocaine, bupivacaine, lidocaine, mepivacaine, prilocaine, procaine, ropivacaine, tetracaine |
| Antineoplastic agents | cyclophosphamide, flutamide, hydroxyurea, ifosfamide, rasburicase |
| Antibiotics | dapsone, nitrofurantoin, para-aminosalicylic acid, sulfonamides |
| Antimalarials | chloroquine, primaquine |
| Anticonvulsants | phenobarbital, phenytoin, sodium valproate |
| Other drugs | acetaminophen, metoclopramide, quinine, sulfasalazine |
Warnings for Posimir
Included as part of the "PRECAUTIONS" Section
Precautions for Posimir
Risk Of Potential Adverse Embolic Effects Resulting From Inadvertent intra vascular Injection
Inadvertent intra vascular injection could cause POSIMIR droplets to be deposited in the pulmonary and other capillary beds. Administer POSIMIR into the subacromial space at the end of arthroscopic shoulder surgery. Direct arthroscopic visualization must be used to confirm proper placement of the needle tip before injectingPOSIMIR.
Risk Of Joint Cartilage Necrosis With Unapproved Intra-Articular Use
The safety and effectiveness of POSIMIR in surgical procedures other than subacromial decompression have not been established, and POSIMIR is not approved for use via intra-articular injection. A study evaluating the effects of POSIMIR and POSIMIR vehicle in dogs following an intra-articular administration demonstrated joint cartilage necrosis [ see Nonclinical Toxicology].
Risk Of Systemic Toxicity
Unintended intra vascular injection of POSIMIR may be associated with systemic toxicities, including CNS or cardiorespiratory depression and coma, progressing ultimately to respiratory arrest. Direct arthroscopic visualization must be used to confirm proper placement of the needle tip in the subacromial space before injecting POSIMIR.
The safety and effectiveness of bupivacaine depend on proper dosage, correct technique, adequate precautions, and readiness for emergencies. Careful and constant monitoring of cardiovascular and respiratory (adequacy of ventilation) vital signs and the patient's state of consciousness should be performed after injection of bupivacaine.
Possible early warning signs of central nervous system (CNS) toxicity are restlessness, anxiety, incoherent speech, lightheadedness, numbness and tingling of the mouth and lips, metallic taste, tinnitus, dizziness, blurred vision, tremors, twitching, CNS depression, or drowsiness. Delay in proper management of systemic toxicity, under ventilation from any cause, and/or altered sensitivity may lead to the development of acidosis, cardiac arrest, and, possibly, death.
Avoid additional use of local anesthetics within 168 hours following administration of POSIMIR. Injection of repeated doses of bupivacaine may cause significant increases in plasma levels due to slow accumulation of the drug or its metabolites, or to slow metabolic degradation. Tolerance to elevated blood levels varies with the status of the patient. Consider increased monitoring for systemic toxicity in debilitated, elderly, or acutely ill patients.
Methemoglobinemia
Cases of methemoglobinemia have been reported in association with local anesthetic use. Although all patients are at risk for methemoglobinemia, patients with glucose-6-phosphate dehydrogenase deficiency, congenital or idiopathic methemoglobinemia, cardiac or pulmonary compromise, infants under 6 months of age, and concurrent exposure to oxidizing agents or their metabolites are more susceptible to developing clinical manifestations of the condition [ see DRUG INTERACTIONS]. If local anesthetics must be used in these patients, close monitoring for symptoms and signs of methemoglobinemia is recommended.
Signs of methemoglobinemia may occur immediately or may be delayed some hours after exposure, and are characterized by a cyanotic skin discoloration and/or abnormal coloration of the blood. Methemoglobin levels may continue to rise; therefore, immediate treatment is required to avert more serious central nervous system and cardiovascular adverse effects, including seizures, coma, arrhythmias, and death. Discontinue any oxidizing agents. Depending on the severity of the signs and symptoms, patients may respond to supportive care, i.e., oxygen therapy, hydration. A more severe clinical presentation may require treatment with methylene blue, exchange transfusion, or hyperbaric oxygen.
Chondrolysis With Intra-Articular Infusion Of Local Anesthetics
Intra-articular infusions of local anesthetics including bupivacaine following arthroscopic and other surgical procedures is an unapproved use, and there have been post-marketing reports of chondrolysis in patients receiving such infusions. The majority of reported cases of chondrolysis have involved the shoulder joint; cases of gleno-humeral chondrolysis have been described in pediatric patients and adult patients following intra-articular infusions of local anesthetics with and without epinephrine for periods of 48 to 72 hours. There is insufficient information to determine whether shorter infusion periods are associated with chondrolysis. The time of onset of symptoms, such as joint pain, stiffness, and loss of motion can be variable, but may begin as early as the second month after surgery. Currently, there is no effective treatment for chondrolysis; patients who have experienced chondrolysis have required additional diagnostic and therapeutic procedures and some required arthroplasty or shoulder replacement.
Risk Of Toxicity In Patients With Hepatic Impairment
Because amide local anesthetics such as bupivacaine are metabolized by the liver, consider reduced dosing and increased monitoring for bupivacaine systemic toxicity in patients with moderate to severe hepatic impairment who are treated with POSIMIR [see Use In Specific Populations].
Risk Of Use In Patients With Impaired Cardiovascular Function
Care should be taken when considering the use of POSIMIR in patients with impaired cardiovascular function (e.g., hypotension, heart block) because they may be less able to compensate for functional changes associated with the prolongation of AV conduction produced by bupivacaine. Consider reduced dosing. Monitor patients closely for blood pressure, heart rate, and ECG changes.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment Of Fertility
Carcinogenesis
Long-term studies in animals to evaluate the carcinogenic potential of bupivacaine hydrochloride have not been conducted.
Mutagenesis
Bupivacaine was negative in the in vitro bacterial reverse mutation assay (Ames assay), the in vitro chromosomal aberration assay (human peripheral blood lymphocytes), and the in vivo rat micronucleus assay.
Impairment Of Fertility
The effect of bupivacaine on fertility has not been determined.
Use In Specific Populations
Pregnancy
Risk Summary
There are no studies conducted with POSIMIR in pregnant women. In animal studies, embryo-fetal lethality was noted when bupivacaine was administered subcutaneously to pregnant rabbits during organogenesis at 0.6 times the maximum recommended human dose of POSIMIR at 660 mg bupivacaine. Decreased pup survival was observed in a rat pre- and post-natal development study (dosing from implantation through weaning) at 0.6 times the maximum recommended human dose of POSIMIR at 660 mg bupivacaine. Based on animal data, advise pregnant women of the potential risks to a fetus. [see Data]
The background risk of major birth defects and miscarriage for the indicated population is unknown. However, the background risk in the U.S. general population of major birth defects is 2%-4% and of miscarriage is 15%-20% of clinically recognized pregnancies.
Clinical Considerations
Labor or Delivery
Bupivacaine is contraindicated for obstetrical paracervical block anesthesia. While POSIMIR has not been studied with this technique, the use of bupivacaine for obstetrical paracervical block anesthesia has resulted in fetal bradycardia and death. Bupivacaine can rapidly cross the placenta, and when used for epidural, caudal, or pudendal block anesthesia, can cause varying degrees of maternal, fetal, and neonatal toxicity (POSIMIR is not indicated for these uses). The incidence and degree of toxicity depend upon the procedure performed, the type, and amount of drug used, and the technique of drug administration. Adverse reactions in the parturient, fetus, and neonate involve alterations of the central nervous system, peripheral vascular tone, and cardiac function.
Data
Animal Data
Bupivacaine hydrochloride produced developmental toxicity when administered subcutaneously to pregnant rats and rabbits at clinically relevant doses.
Bupivacaine hydrochloride was administered subcutaneously to rats at doses of 4.4, 13.3, & 40 mg/kg and to rabbits at doses of 1.3, 5.8, & 22.2 mg/kg during the period of organogenesis (implantation to closure of the hard palate). The high doses are approximately 0.6 times the daily maximum recommended human dose (MRHD) of 660 mg/day on a mg/m2 body surface area (BSA) basis. No embryo-fetal effects were observed in rats at the high dose which caused increased maternal lethality. An increase in embryo-fetal deaths was observed in rabbits at the high dose in the absence of maternal toxicity with the fetal No Observed Adverse Effect Level representing approximately 0.2 times the MRHD on a BSA basis.
In a rat pre- and post-natal developmental study (dosing from implantation through weaning) conducted at subcutaneous doses of 4.4, 13.3, & 40 mg/kg, decreased pup survival was observed at the high dose. The high dose is approximately 0.6 times the daily MRHD of 660 mg/day on a BSA basis.
Lactation
Risk Summary
POSIMIR has not been studied in nursing mothers. Bupivacaine can persist in plasma for up to 168 hours [see Clinical Studies] and benzyl alcohol, a POSIMIR excipient, for up to 12 hours after POSIMIR administration. Both bupivacaine and benzyl alcohol have been reported to be excreted in human milk. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for POSIMIR and any potential adverse effects on the breastfed infant from POSIMIR or from the underlying maternal condition.
Pediatric Use
Safety and effectiveness of POSIMIR in pediatric patients below the age of 18 have not been established.
Geriatric Use
Of the total number of patients enrolled in the POSIMIR clinical studies (N=1463), 167 patients were older than 65 years and 32 patients were older than 75 years.
In bupivacaine clinical studies, differences in various pharmacokinetic parameters have been observed between elderly and younger adult patients. Bupivacaine is known to be substantially excreted by the kidney, and the risk of adverse reactions to bupivacaine may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function. Elderly patients may require lower doses of POSIMIR. Consider increased monitoring for local anesthetic systemic toxicity when administering POSIMIR to elderly patients.
Hepatic Impairment
Because amide-type local anesthetics, such as bupivacaine, are metabolized by the liver, these drugs should be used with caution in patients with hepatic impairment. Patients with severe hepatic impairment, because of their inability to metabolize local anesthetics normally, are at a greater risk of developing toxic plasma concentrations, and potentially local anesthetic systemic toxicity. Care should be taken when considering the use of POSIMIR in patients with impaired hepatic function and consider reduced dosing. Consider increased monitoring for local anesthetic systemic toxicity in patients with moderate to severe hepatic impairment when administering POSIMIR.
Renal Impairment
Bupivacaine is known to be substantially excreted by the kidney, and the risk of adverse reactions to this drug may be greater in patients with impaired renal function. Care should be taken when considering the use of POSIMIR in patients with impaired renal function. Consider increased monitoring for local anesthetic systemic toxicity when administering POSIMIR to patients with impaired renal function.
Overdose Information for Posimir
Acute emergencies from local anesthetics are generally related to high plasma concentrations encountered during therapeutic use of local anesthetics or to unintended intravascular injection of local anesthetic solution [see WARNINGS AND PRECAUTIONS].
In the clinical study program, a maximum plasma concentration (Cmax) of 2850 ng/mL was reported. No apparent bupivacaine-related adverse reactions or clinical sequelae were observed in patients with high bupivacaine plasma concentrations.
Contraindications for Posimir
POSIMIR is contraindicated in:
- Patients with a known hypersensitivity (e.g. anaphylactic reactions and serious skin reactions) to any amide local anesthetic, or other components of POSIMIR.
- Patients undergoing obstetrical paracervical block anesthesia. The use of bupivacaine HCl with this technique has resulted in fetal bradycardia and death.
Clinical Pharmacology for Posimir
Mechanism Of Action
Bupivacaine blocks the generation and the conduction of nerve impulses, presumably by increasing the threshold for electrical excitation in the nerve, by slowing the propagation of the nerve impulse, and by reducing the rate of rise of the action potential. In general, the progression of anesthesia is related to the diameter, myelination and conduction velocity of affected nerve fibers. Clinically, the order of loss of nerve function is as follows: (1) pain, (2) temperature, (3) touch, (4) proprioception, and (5) skeletal muscle tone.
Pharmacodynamics
Systemic absorption of bupivacaine produces effects on the cardiovascular and central nervous systems. At blood concentrations achieved with therapeutic doses, changes in cardiac conduction, excitability, refractoriness, contractility, and peripheral vascular resistance are minimal. However, toxic blood concentrations depress cardiac conduction and excitability, which may lead to atrioventricular block, ventricular arrhythmias and to cardiac arrest, sometimes resulting in fatalities. In addition, myocardial contractility is depressed and peripheral vasodilation occurs, leading to decreased cardiac output and arterial blood pressure. These cardiovascular changes are more likely to occur after unintended intravascular injection of liquid formulations of bupivacaine.
Following systemic absorption, local anesthetics can produce central nervous system stimulation, depression or both. Apparent central stimulation is usually manifested as restlessness, tremors and shivering, progressing to convulsions, followed by depression and coma, progressing ultimately to respiratory arrest. However, the local anesthetics have a primary depressant effect on the medulla and on higher centers. The depressed stage may occur without a prior excited stage.
Pharmacokinetics
Infiltration of POSIMIR into the surgical wound results in plasma levels of bupivacaine that can persist for 168 hours. Systemic plasma levels of bupivacaine following administration of POSIMIR are not correlated with local efficacy.
Absorption
The rate of systemic absorption of bupivacaine is dependent upon the total dose of drug administered, the route of administration, and the vascularity of the administration site.
Pharmacokinetic parameters of bupivacaine are shown in Table 10 after single-dose infiltration of POSIMIR in arthroscopic subacromial decompression surgical procedure.
Table 10. Summary of Pharmacokinetic Parameters for Bupivacaine after Administration of a Single Dose of POSIMIR 5 mL (660 mg)
| Surgical Procedure | Cmax (ng/mL) |
AUC0-t# (h·ng/mL) | Tmax (h) |
T1/2 (h) |
|
| Arthroscopic subacromial decompression Study 1* | N | 36 | 36 | 36 | 36 |
| Mean | 593 | 19395 | 5.9^ | 16.4 | |
| SD | 299 | 12056 | 1.0-24.0^ | 5.1 | |
| Arthroscopic subacromial decompression | N | 18 | 18 | 18 | 18 |
| Mean | 1006 | 47015 | 8.0^ | 26.1 | |
| SD | 454 | 20040 | 2.1-26.9^ | 8.2 | |
| #t = last sampling time ^ Median / Range (min-max) * Drug leakage from the surgical site was suspected. |
|||||
Distribution
Depending upon the route of administration, bupivacaine is distributed to some extent to all body tissues, with high concentrations found in highly perfused organs such as the liver, lungs, heart, and brain.
Bupivacaine appears to cross the placenta by passive diffusion. The rate and degree of diffusion is governed by (1) the degree of plasma protein binding, (2) the degree of ionization, and (3) the degree of lipid solubility. Fetal/maternal ratios of local anesthetics appear to be inversely related to the degree of plasma protein binding, because only the free, unbound drug is available for placental transfer. Bupivacaine with a high protein binding capacity (95%) has a low fetal/maternal ratio (0.2 to 0.4). The extent of placental transfer is also determined by the degree of ionization and lipid solubility of the drug. Lipid soluble, non-ionized drugs such as bupivacaine readily enter the fetal blood from the maternal circulation.
Elimination
The mean half-life of bupivacaine after POSIMIR administration in adults who underwent arthroscopic subacromial decompression range from 16.4 to 26.1 hours.
Metabolism
Amide-type local anesthetics such as bupivacaine are metabolized primarily in the liver via conjugation with glucuronic acid. Pipecoloxylidine is the major metabolite of bupivacaine. The elimination of drug from tissue distribution depends largely upon the availability of binding sites in the circulation to carry it to the liver where it is metabolized.
Excretion
The kidney is the main excretory organ for most local anesthetics and their metabolites. Urinary excretion is affected by urinary perfusion and factors affecting urinary pH. Only 6% of bupivacaine is excreted unchanged in the urine.
Specific Populations
Hepatic Impairment
Pharmacokinetics of POSIMIR have not been evaluated in patients with hepatic impairment. [see WARNINGS AND PRECAUTIONS and Use In Specific Populations].
Renal Impairment
Pharmacokinetics of POSIMIR have not been evaluated in patients with renal impairment. [see Use In Specific Populations].
Geriatric Patients
Pharmacokinetics of POSIMIR have not been evaluated in geriatric patients.
Elderly patients have exhibited higher peak plasma concentrations than younger patients following administration of bupivacaine HCl. The total plasma clearance was decreased in these patients [see Use In Specific Populations].
Animal Toxicology And/Or Pharmacology
Necrosis of the joint cartilage was observed following intra-articular injection of a single dose of POSIMIR or POSIMIR vehicle in the dog model.
Clinical Studies
The effectiveness of POSIMIR was evaluated in ten adequate and well-controlled studies in patients undergoing open and laparoscopic abdominal procedures, abdominal hysterectomy, inguinal hernia repair, and open and arthroscopic shoulder procedures. Adequate evidence of effectiveness was demonstrated in one of three studies conducted in patients undergoing shoulder surgery (described in detail below) and was not demonstrated in any soft tissue procedure evaluated.
Study 1
Study 1 was a randomized, multicenter, assessor blinded, placebo-controlled (vehicle) clinical trial in 107 patients undergoing arthroscopic subacromial decompression surgery with an intact rotator cuff. Associated procedures included inspection of the glenohumeral joint, distal clavicle excision, bursectomy, synovectomy, removal of loose body, resection of coracoacromial ligament and subacromial spurs, rotator cuff debridement, and minor debridement of articular cartilage. There were no open surgical procedures performed during this study. The mean age was 50 years (range 21 to 70 years), 60% of treated patients were female, 96% were White, 2% were Hispanic, 1% were Asian, and 1% were Other.
Patients were randomized 2:1:1 to receive POSIMIR, vehicle placebo, or bupivacaine HCl 50 mg, and all patients received general anesthesia. No analgesic pre-medication or local anesthetics were administered. POSIMIR and vehicle placebo were administered under direct arthroscopic visualization as single injections into the subacromial space through one of the arthroscopic portals at the end of the surgery. Bupivacaine HCl 50 mg was administered subacromially as a single dose. Post-operatively, patients received acetaminophen 500 mg or 1000 mg every six hours, depending on body weight, through 72 hours, and were allowed morphine rescue medication as needed, either 2 mg IV or 10 mg orally. Pain intensity was rated by the patients using a 0 to 10 numerical rating scale (NRS) at multiple time points up to72 hours.
The primary outcome measures were the normalized area under the curve (nAUC) of mean pain intensity on movement scores collected at specified intervals over the first 72 hours after surgery and total opioid rescue analgesia (IV morphine-equivalent dose) through 72 hours. In this clinical study, POSIMIR 5 mL demonstrated a significant reduction in mean pain intensity compared with placebo of 1.3 points on a 0 to 10 NRS scale over 72 hours ( Figure 1 ).
Figure 1. Mean Pain Intensity on Movement Through 72 Hours After Surgery, Subacromial Decompression Study 1
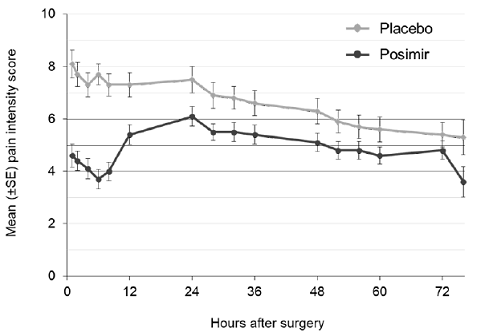 |
The median total use of opioid rescue analgesia (IV morphine equivalent dose) from 0 to 72 hours for the POSIMIR treatment group (4 mg) was statistically lower than for the placebo treatment group (12 mg). The median use of opioid rescue analgesia in the bupivacaine treatment group was 8 mg.
Study 2
Study 2 was a randomized, double-blind, placebo-controlled (vehicle) clinical trial in 60 patients undergoing arthroscopic subacromial decompression, inspection of glenohumeral joint, synovectomy, removal of loose body, minor debridement of articular cartilage, minor debridement or minor repair of rotator cuff, open distal clavicle excision, bursectomy, and resection of coracoacromial ligament and subacromial spurs. The mean age was 48 years (range 27 to 68 years), 55% of treated patients were female, 95% were White, 2% were Asian, and 2% were Other.
Patients were randomized 2:1 to receive POSIMIR or vehicle placebo, and all patients received general anesthesia. Post-operatively, patients were allowed morphine rescue medication as needed, either 3 mg IV or 10 mg to 15 mg orally, or acetaminophen. Pain intensity was rated by the patients using a 0 to 10 numerical rating scale (NRS) at multiple time points up to 72 hours.
The primary outcome measures were mean pain intensity on movement AUC through 72 hours and total opioid rescue analgesia (IV morphine-equivalent dose) through 72 hours. There was no statistically significant difference in either primary endpoint between the POSIMIR and vehicle placebo treatment groups ( Figure 2 ).
Figure 2: Mean Pain Intensity on Movement Through 72 Hours After Surgery, Subacromial Decompression Study 2
 |
Study 3
Study 3 was a randomized, double-blind, placebo-controlled (vehicle) and open-label PK clinical trial in 92 patients undergoing a variety of shoulder surgical procedures, including rotator cuff repair, subacromial decompression, glenoid labrum repair or debridement, and biceps tendon repair. The majority of patients underwent arthroscopic procedures; however, six patients underwent a combination of arthroscopic and open procedures. The mean age was 54 years (range 20 to 82 years), 59% of treated patients were male, 87% were White, 8% were African American, 3% were Other, and 2% were Asian.
An equal number of patients were randomized to two cohorts. The routes of POSIMIR or vehicle placebo administration in Cohort 1 were subacromial or subcutaneous injection or a combination. In Cohort 2, POSIMIR or vehicle placebo was administered via injection under direct arthroscopic visualization into the subacromial space. Surgical procedures were completed either under local or general anesthesia. Post-operatively, patients were allowed morphine IV, oxycodone orally, or acetaminophen orally as needed.
The primary outcome measures were mean pain intensity on movement and at rest through 120 hours and pain control through day seven. There was no statistically significant difference in either primary endpoint between POSIMIR and vehicle placebo treatment groups ( Figure 3 ).
Figure 3: Mean Pain Intensity on Movement Through 120 Hours After Surgery, Subacromial Decompression Study 3
 |
Patient Information for Posimir
Patients should be informed in advance that bupivacaine-containing products can cause temporary loss of sensation or motor activity in the area of infiltration. The physician should discuss other information including adverse reactions in the POSIMIR package insert with their patients.
From 

Report Problems to the Food and Drug Administration
You are encouraged to report negative side effects of prescription drugs to the FDA. Visit the FDA MedWatch website or call 1-800-FDA-1088.