Description for VoSpire ER
Albuterol extended-release tablets contain albuterol sulfate, the racemic form of albuterol and a relatively selective beta2-adrenergic bronchodilator, in an extended-release formulation. Albuterol sulfate has the chemical name (±) α1-[(tert-butylamino)methyl]-4-hydroxy-m-xylene-α, α-diol sulfate (2:1) (salt), and the following structural formula:
 |
Albuterol sulfate has a molecular weight of 576.7, and the molecular formula is (C13H21NO3)2•H2SO4. Albuterol sulfate is a white crystalline powder, soluble in water and slightly soluble in ethanol.
The World Health Organization recommended name for albuterol base is salbutamol.
Each tablet for oral administration contains 4 mg or 8 mg of albuterol as 4.8 mg or 9.6 mg, respectively, of albuterol sulfate in a cellulosic material that serves as a diffusion-release membrane. In addition each tablet contains the following inactive ingredients: Calcium sulfate, carnauba wax, ethylcellulose, ferric oxide black, hypromellose, ink-thinner XI, lactose monohydrate, magnesium stearate, polyethylene glycol, propylene glycol, shellac, stearic acid, titanium dioxide, triacetin, D&C Yellow #10, (4 mg only) and FD&C Blue #1 (4 mg only).
Uses for VoSpire ER
Albuterol extended-release tablets are indicated for the relief of bronchospasm in adults and children 6 years of age and older with reversible obstructive airway disease.
Dosage for VoSpire ER
The following dosages of albuterol extended-release tablets are expressed in terms of albuterol base:
Usual Dosage
Adults and Children over 12 years of age: The usual recommended dosage for adults and pediatric patients over 12 years of age is 8 mg every 12 hours. In some patients, 4 mg every 12 hours may be sufficient.
Children 6 to 12 years of age: The usual recommended dosage for children 6 through 12 years of age is 4 mg every 12 hours.
Dosage adjustment in Adults and Children over 12 years of age: In unusual circumstances, such as adults of low body weight, it may be desirable to use a starting dosage of 4 mg every 12 hours and progress to 8 mg every 12 hours according to response.
If control of reversible airway obstruction is not achieved with the recommended doses in patients on otherwise optimized asthma therapy, the doses may be cautiously increased stepwise under the control of the supervising physician to a maximum dose of 32 mg per day in divided doses (i.e., every 12 hours).
Dosage adjustment in Children 6 to 12 years of age: If control of reversible airway obstruction is not achieved with the recommended doses in patients on otherwise optimized asthma therapy, the doses may be cautiously increased stepwise under the control of the supervising physician to a maximum dose of 24 mg per day in divided doses (i.e., every 12 hours).
Switching from oral albuterol, USP products: Patients currently maintained on albuterol tablets, USP or albuterol sulfate syrup can be switched to albuterol extended-release tablets. For example, the administration of one 4 mg albuterol extended-release tablet every 12 hours is comparable to one 2 mg albuterol tablet, USP every 6 hours. Multiples of this regimen up to the maximum recommended daily dose also apply.
Albuterol extended-release tablets must be swallowed whole with the aid of liquids. DO NOT CHEW OR CRUSH THESE TABLETS.
HOW SUPPLIED
Albuterol Extended-Release Tablets, equivalent to 4 mg and 8 mg of Albuterol:
4 mg - Green, round, coated tablets in bottles of 100.
(NDC 68774-600-01)
Printed V on one side and 4 on the other side in black ink.
8 mg - White, round, coated tablets in bottles of 100.
(NDC 68774-601-01)
Printed V on one side and 8 on the other side in black ink.
Dispense in a well-closed, light-resistant container as defined in the USP. Replace cap securely after each opening. Store at 20°-25°C (68°-77°F) [See USP Controlled Room Temperature].
Manufactured by: PLIVA® Inc. East Hanover, NJ 07936. For: DAVA Pharmaceuticals, Inc. Fort Lee, New Jersey 07024, USA. Rev. 9/05. FDA Rev date: 09/30/02
Side Effects for VoSpire ER
The adverse reactions to albuterol are similar in nature to reactions to other sympathomimetic agents. The most frequent adverse reactions to albuterol are nervousness, tremor, headache, tachycardia, and palpitations. Less frequent adverse reactions are muscle cramps, insomnia, nausea, weakness, dizziness, drowsiness, flushing, restlessness, irritability, chest discomfort, and difficulty in micturition.
Rare cases of urticaria, angioedema, rash, bronchospasm, and oropharyngeal edema have been reported after the use of albuterol.
In addition, albuterol, like other sympathomimetic agents, can cause adverse reactions such as hypertension, angina, vomiting, vertigo, central nervous system stimulation, unusual taste, and drying or irritation of the oropharynx.
In controlled clinical trials of adult patients conducted in the United States, the following incidence of adverse events was reported:
| Event | Albuterol Extended-Release Tablets (n=330) |
Theophylline (n=197) |
Other Beta-agonists (n=20) |
Placebo (n=178) |
| Tremor | 24.2% | 6.1% | 35.0% | 1.1% |
| Headache | 18.8% | 26.9% | 35.0% | 20.8% |
| Nervousness | 8.5% | 5.1% | 10.0% | 2.8% |
| Nausea/Vomiting | 4.2% | 19.8% | 5.0% | 3.9% |
| Tachycardia | 2.7% | 0.5% | 5.0% | 0% |
| Muscle Cramps | 2.7% | 0.5% | 5.0% | 0.6% |
| Palpitations | 2.4% | 0.5% | 0% | 1.1% |
| Insomnia | 2.4% | 6.1% | 0% | 1.7% |
| Dizziness | 1.5% | 2.0% | 0% | 5.1% |
| Somnolence | 0.3% | 1.0% | 0% | 0.6% |
A trend was observed among patients treated with albuterol extended-release tablets toward increasing frequency of muscle cramps with increasing patient age (12-20 years, 1.2%; 21-30 years, 2.6%; 31-40 years, 6.9%; 41-50 years, 6.9%), compared with no such events in the placebo group. Also observed was an increasing frequency of tremor with increasing patient age (12-20 years, 29.4%; 21-30 years, 29.9%; 31-40 years, 27.6%; 41-50 years, 37.9%), compared to 2.9% or less in the placebo group.
The reactions are generally transient in nature, and it is usually not necessary to discontinue treatment with albuterol extended-release tablets.
Drug Interactions for VoSpire ER
The concomitant use of albuterol extended-release tablets and other oral sympath-omimetic agents is not recommended since such combined use may lead to deleterious cardiovascular effects. This recommendation does not preclude the judicious use of an aerosol bronchodilator of the adrenergic stimulant type in patients receiving albuterol extended-release tablets. Such concomitant use, however, should be individualized and not given on a routine basis. If regular coadministration is required, then alternative therapy should be considered.
Monoamine Oxidase Inhibitors or Tricyclic Antidepressants: Albuterol should be administered with extreme caution to patients being treated with monoamine oxidase inhibitors or tricyclic antidepressants, or within 2 weeks of discontinuation of such agents, because the action of albuterol on the vascular system may be potentiated.
Beta Blockers: Beta-adrenergic receptor blocking agents not only block the pulmonary effect of beta-agonists, such as albuterol extended-release tablets, but may produce severe bronchospasm in asthmatic patients. Therefore, patients with asthma should not normally be treated with beta-blockers. However, under certain circumstances, e.g., as prophylaxis after myocardial infarction, there may be no acceptable alternatives to the use of beta-adrenergic blocking agents in patients with asthma. In this setting, cardio-selective beta-blockers could be considered, although they should be administered with caution.
Diuretics: The ECG changes and/or hypokalemia that may result from the administration of non potassium-sparing diuretics (such as loop or thiazide diuretics) can be acutely worsened by beta-agonists, especially when the recommended dose of the beta-agonist is exceeded. Although the clinical significance of these effects is not known, caution is advised in the coadministration of beta-agonists with non potassium-sparing diuretics.
Digoxin: Mean decreases of 16% to 22% in serum digoxin levels were demonstrated after single dose intravenous and oral administration of albuterol, respectively, to normal volunteers who had received digoxin for 10 days. The clinical significance of these findings for patients with obstructive airway disease who are receiving albuterol and digoxin on a chronic basis is unclear. Nevertheless, it would be prudent to carefully evaluate the serum digoxin levels in patients who are currently receiving digoxin and albuterol.
Warnings for VoSpire ER
Immediate hypersensitivity reactions may occur after administration of albuterol, as demonstrated by rare cases of urticaria, angioedema, rash, bronchospasm, and oropharyngeal edema.
Cardiovascular Effects: Albuterol extended-release tablets, like all other beta-adrenergic agonists, can produce a clinically significant cardiovascular effect in some patients, as measured by pulse rate, blood pressure, and/or symptoms. Although such effects are uncommon after administration of albuterol extended-release tablets at recommended doses, if they occur, the drug may need to be discontinued. In addition, beta-agonists have been reported to produce electrocardiogram (ECG) changes, such as flattening of the T wave, prolongation of the QTc interval, and ST segment depression. The clinical significance of these findings is unknown. Therefore, albuterol extended-release tablets, like all sympathomimetic amines, should be used with caution in patients with cardiovascular disorders, especially coronary insufficiency, cardiac arrhythmias, and hypertension.
Deterioration of Asthma: Asthma may deteriorate acutely over a period of hours or chronically over several days or longer. If the patient needs more doses of albuterol extended-release tablets than usual, this may be a marker of destabilization of asthma and requires reevaluation of the patient and the treatment regimen, giving special consideration to the possible need for anti-inflammatory treatment; e.g., corticosteroids.
Use of Anti-Inflammatory Agents: The use of beta adrenergic agonist bronchodilators alone may not be adequate to control asthma in many patients. Early consideration should be given to adding anti-inflammatory agents; e.g., corticosteroids.
Paradoxical Bronchospasm: Albuterol extended-release tablets can produce paradoxical bronchospasm, which may be life threatening. If paradoxical bronchospasm occurs, albuterol extended-release tablets should be discontinued immediately and alternative therapy instituted.
Rarely, erythema multiforme and Stevens-Johnson syndrome have been associated with the administration of oral albuterol in children.
Precautions for VoSpire ER
General: Albuterol, as with all sympathomimetic amines, should be used with caution in patients with cardiovascular disorders, especially coronary insufficiency, cardiac arrhythmias, and hypertension; in patients with convulsive disorders, hyperthyroidism, or diabetes mellitus; and in patients who are unusually responsive to sympathomimetic amines. Clinically significant changes in systolic and diastolic blood pressure have been seen and could be expected to occur in some patients after use of any beta-adrenergic bronchodilator.
In controlled clinical trials in adults, patients treated with albuterol extended-release tablets had increases in selected serum chemistry values and decreases in selected hematologic values. Increases in SGPT were more frequent among patients treated with albuterol extended-release tablets (12 of 247 patients, 4.9%) than among the theophylline (6 of 188 patients, 3.2%) and placebo (1 of 138 patients, 0.7%) groups. Increases in serum glucose concentration were also more frequent among patients treated with albuterol extended-release tablets (23 of 234 patients, 9.8%) than among theophylline (11 of 173 patients, 6.45%) and placebo (3 of 129 patients, 2.3%) groups. Increases in SGOT were also more frequent among patients treated with albuterol extended-reIease tablets (10 of 248 patients, 4%) and theophylline (5 of 193, 2.6%) than among patients treated with placebo. Decreases in white blood cell counts were more frequent in patients treated with albuterol extended-release tablets (10 of 247 patients, 4%) compared with patients receiving theophylline (2 of 185 patients, 1.1%) and patients receiving placebo (1 of 141 patients, 0.7%). Decreases in hemoglobin and hematocrit were more frequent in patients receiving albuterol extended-release tablets (16 of 228 patients, 7.0%, and 17 of 230 patients, 7.4%, respectively) than in patients receiving theophylline (5 of 171 patients, 2.9%, and 9 of 173 patients, 5.2%, respectively) and patients receiving placebo (5 of 129 patients, 3.9%, and 3 of 132 patients, 2.3%, respectively). The clinical significance of these results is unknown.
Large doses of intravenous albuterol have been reported to aggravate pre-existing diabetes mellitus and ketoacidosis. As with other beta-agonists, albuterol may produce significant hypokalemia in some patients, possibly through intracellular shunting, which has the potential to produce adverse cardiovascular effects. The decrease is usually transient, not requiring supplementation.
Carcinogenesis, Mutagenesis, Impairment Of Fertility: In a 2-year study in Sprague-Dawley rats, albuterol sulfate caused a significant dose-related increase in the incidence of benign leiomyomas of the mesovarium at dietary doses of 2.0, 10, and 50 mg/kg, (approximately 1/2, 3, and 15 times, respectively, the maximum recommended daily oral dose for adults on a mg/m2 basis, or, approximately 2/5, 2, and 10 times, respectively, the maximum recommended daily oral dose for children on a mg/m2 basis). In another study this effect was blocked by the coadministration of propranolol, a non-selective beta-adrenergic antagonist. In an 18 month study in CD-1 mice, albuterol sulfate showed no evidence of tumorigenicity at dietary doses of up to 500 mg/kg (approximately 65 times the maximum recommended daily oral dose for adults on a mg/m2 basis, or, approximately 50 times the maximum recommended daily oral dose for children on a mg/m2 basis). In a 22 month study in the Golden hamster, albuterol sulfate showed no evidence of tumorigenicity at dietary doses of 50 mg/kg, (approximately 7 times the maximum recommended daily oral dose for adults and children on a mg/m2 basis).
Albuterol sulfate was not mutagenic in the Ames test with or without metabolic activation using tester strains S. typhimurium TA 1537, TA 1538, and TA98 or E. coli WP2, WP2uvrA, and WP67. No forward mutation was seen in yeast strainS. cerevisiae S9 nor any mitotic gene conversion in yeast strain S. cerevisiae JD1 with or without metabolic activation. Fluctuation assays in S. typhimurium TA98 and E. coli WP2, both with metabolic activation, were negative. Albuterol sulfate was not clastogenic in a human peripheral lymphocyte assay or in an AH1 strain mouse micronucleus assay at intraperitoneal doses of up to 200 mg/kg.
Reproduction studies in rats demonstrated no evidence of impaired fertility at oral doses up to 50 mg/kg, (approximately 15 times the maximum recommended daily oral dose for adults on a mg/m2 basis).
Pregnancy: Teratogenic Effects: Pregnancy Category C: Albuterol Sulfate has been shown to be terato-genic in mice. A study in CD-1 mice at subcutaneous (SC) doses of 0.025, 0.25, and 2.5 mg/kg, (approximately 3/1000, 3/100, and 3/10 times the maximum recommended daily oral dose for adults on a mg/m2 basis), showed cleft palate formation in 5 of 111 (4.5%) fetuses at 0.25 mg/kg and in 10 of 108 (9.3%) fetuses at 2.5 mg/kg. The drug did not induce cleft palate formation at the lowest dose, 0.025 mg/kg. Cleft palate also occurred in 22 of 72 (30.5%) fetuses of females treated with 2.5 mg/kg, of isoproterenol (positive control) subcutaneously (approximately 3/10 times the maximum recommended daily oral dose for adults on a mg/m2 basis). A reproduction study in Stride Dutch rabbits revealed cranioschisis in 7/19 fetuses (37%) when albuterol sulfate was administered orally at a 50 mg/kg dose, (approximately 25 times the maximum recommended daily oral dose for adults on a mg/m2 basis).
There are no adequate and well-controlled studies in pregnant women. Albuterol should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
During worldwide marketing experience, various congenital anomalies, including cleft palate and limb defects, have been rarely reported in the offspring of patients being treated with albuterol. Some of the mothers were taking multiple medications during their pregnancies. No consistent pattern of defects can be discerned, and a relationship between albuterol use and congenital anomalies has not been established.
Labor and Delivery: Because of the potential for beta-agonist interference with uterine contractility, use of albuterol extended-release tablets for relief of bronchospasm during labor should be restricted to those patients in whom the benefits clearly outweigh the risks.
Tocolysis: Albuterol has not been approved for the management of pre-term labor. The benefit:risk ratio when albuterol is administered for tocolysis has not been established. Serious adverse reactions, including pul- monary edema, have been reported during or following treatment of premature labor with beta2-agonists, including albuterol.
Nursing Mothers: It is not known whether albuterol is excreted in human milk. Because of the potential for tumorigenicity shown for albuterol in animal studies, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
Pediatric Use: The safety and effectiveness of albuterol extended-release tablets have been established in pediatric patients 6 years of age or older. Use of albuterol extended-release tablets in these age groups is supported by evidence from adequate and well-controlled studies of albuterol extended-release tablets in adults; the likelihood that the disease course, pathophysiology, and the drug's effect in pediatric and adult patients are substantially similar; the established safety and effectiveness of immediate release albuterol tablets in pediatric patients 6 years of age and older; and clinical trials that support the safety of albuterol extended-release tablets in pediatric patients over 6 years of age. The recommended dose of albuterol extended-release tablets for the pediatric population is based upon the recommended pediatric dosing of immediate-release albuterol tablets and pharmacokinetic studies in adults showing comparable bioavailability at steadystate dosing and reduced bioavailability after single dose administration. Safety and effectiveness in pediatric patients below 6 years of age have not been established.
Overdose Information for VoSpire ER
The expected symptoms with overdosage are those of excessive beta-adrenergic stimulation and/or occurrence or exaggeration of any of the symptoms listed under ADVERSE REACTIONS; e.g., seizures, angina, hypertension or hypotension, tachycardia with rates up to 200 beats per minute, arrhythmias, nervousness, headache, tremor, dry mouth, palpitation, nausea, dizziness, fatigue, malaise, and insomnia. Hypokalemia may also occur. As with all sympathomimetic aerosol medications, cardiac arrest and even death may be associated with abuse of albuterol extended-release tablets.
Treatment consists of discontinuation of albuterol extended-release tablets together with appropriate symptomatic therapy. The judicious use of a cardioselective beta-receptor blocker may be considered, bearing in mind that such medication can produce bronchospasm. There is insufficient evidence to determine if dialysis is beneficial for overdosage of albuterol extended-release tablets.
The oral median lethal dose of albuterol sulfate in mice is greater than 2000 mg/kg, (approximately 250 times the maximum recommended daily oral dose for adults on a mg/m2 basis, or, approximately 200 times the maximum recommended daily oral dose for children on a mg/m2 basis). In mature rats, the subcutaneous median lethal dose of albuterol sulfate is approximately 450 mg/kg (approximately 110 times the maximum recommended daily oral dose for adults on a mg/m2 basis, or, approximately 90 times the maximum recommended daily oral dose for children on a mg/m2 basis). In small young rats, the subcutaneous median lethal dose is approximately 2000 mg/kg, (approximately 500 times the maximum recommended daily oral dose for adults on a mg/m2 basis, or, approximately 400 times the maximum recommended daily oral dose for children on a mg/m2 basis).
Contraindications for VoSpire ER
Albuterol extended-release tablets are contraindicated in patients with a history of hypersensitivity to albuterol or any of its components.
Clinical Pharmacology for VoSpire ER
In vitro studies and in vivo pharmacologic studies have demonstrated that albuterol has a preferential effect on beta2-adrenergic receptors compared with isoproterenol. While it is recognized that beta2-adrenergic receptors are the predominant receptors in bronchial smooth muscle, data indicates that there is a population of beta2-receptors in the human heart existing in a concentration between 10% and 50%. The precise function of these receptors has not been established. (See WARNINGS).
The pharmacologic effects of beta-adrenergic agonist drugs, including albuterol, are at least in part attributable to stimulation through beta-adrenergic receptors on intracellular adenyl cyclase, the enzyme that catalyzes the conversion of adenosine triphosphate (ATP) to cyclic-3', 5'-adenosine monophosphate (cyclic AMP). Increased cyclic AMP levels are associated with relaxation of bronchial smooth muscle and inhibition of release of mediators of immediate hypersensitivity from cells, especially from mast cells.
Albuterol has been shown in most controlled clinical trials to have more effect on the respiratory tract, in the form of bronchial smooth muscle relaxation, than isoproterenol at comparable doses while producing fewer cardiovascular effects.
Albuterol is longer acting than isoproterenol in most patients by any route of administration because it is not a substrate for the cellular uptake processes for catecholamines nor for catechol-O-methyl transferase.
Preclinical:Intravenous studies in rats with albuterol sulfate have demonstrated that albuterol crosses the blood-brain barrier and reaches brain concentrations amounting to approximately 5.0% of the plasma concentrations. In structures outside the blood-brain barrier (pineal and pituitary glands), albuterol concentrations were found to be 100 times those in the whole brain.
Studies in laboratory animals (minipigs, rodents, and dogs) have demonstrated the occurrence of cardiac arrhythmias and sudden death (with histologic evidence of myocardial necrosis) when beta-agonists and methylxanthines were administered concurrently. The clinical significance of these findings is unknown.
Pharmacokinetics and Disposition: In a single-dose study comparing one 8 mg albuterol extended-release tablet with two 4 mg immediate-release albuterol tablets, USP in 17 normal adult volunteers, the extent of availability of albuterol extended-release tablets was shown to be about 80% of albuterol tablets, USP with or without food. In addition, lower mean peak plasma concentration and longer time to reach the peak level were observed with albuterol extended-release tablets as compared with albuterol tablets, USP. The single-dose study results also showed that food decreases the rate of absorption of albuterol from albuterol extended-release tablets without altering the extent of bioavailability. In addition, the study indicated that food causes a more gradual increase in the fraction of the available dose absorbed from the extended-release formulation as compared with the fasting condition.
In another single-dose study in adults, 8 mg and 4 mg albuterol extended-release tablets were shown to deliver dose-proportional plasma concentrations in the fasting state. Definitive studies for the effect of food on 4 mg albuterol extended-release tablets have not been conducted. However, since food lowers the rate of absorption of 8 mg albuterol extended-release tablets, it is expected that food reduces the rate of absorption of 4 mg albuterol extended-release tablets also.
Albuterol extended-release tablets have been formulated to provide duration of action of up to 12 hours. In an 8-day, multiple-dose, crossover study, 15 normal adult male volunteers were given 8 mg albuterol extended-release tablets every 12 hours or 4 mg albuterol tablets, USP every 6 hours. Each dose of albuterol extended-release tablets and the corresponding doses of albuterol tablets, USP were administered in the postprandial state. Steady-state plasma concentrations were reached within 2 days for both formulations. Fluctuations (Cmax-Cmin/Caverage) in plasma concentrations were similar for albuterol extended-release tablets administered at 12-hour intervals and albuterol tablets, USP administered every 6 hours. In addition, the relative bioavailability of albuterol extended-release tablets was approximately 100% of the immediate-release tablet at steady state. A summary of these results is shown in the following table:
Mean Values at Steady State
| Cmax (ng/mL) |
Cmin (ng/mL) |
Tmax (h) |
T1/2 (h) |
AUC (ng·h/mL) |
|
| Albuterol Extended-Release Tablets | 13.7 | 8.1 | 6.0 | 9.3 | 134 |
| Albuterol Tablets, USP | 13.9 | 8.1 | 2.6 | 7.2 | 132 |
The mean plasma albuterol concentration versus time data at steady state after the administration of albuterol extended-release tablets 8 mg every 12 hours are displayed in the following graph:
Mean Plasma Albuterol Concentration at Day 8
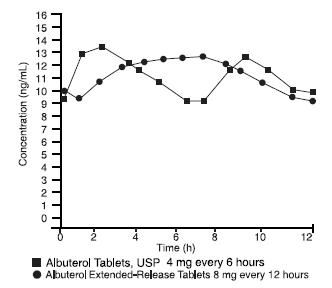 |
Pharmacokinetic studies of 4- and 8-mg albuterol extended-release tablets have not been conducted in pediatric patients. Bioavailability of 4- and 8-mg albuterol extended-release tablets in pediatric patients relative to 2- and 4-mg immediate release albuterol has been extrapolated from adult studies showing comparability at steady-state dosing and reduced bioavailability after single dose administration.
Patient Information for VoSpire ER
Albuterol extended-release tablets must be swallowed whole with the aid of liquids. DO NOT CHEW OR CRUSH THESE TABLETS.
The action of albuterol extended-release tablets should last up to 12 hours or longer. Albuterol extended-release tablets should not be used more frequently than recommended. Do not increase the dose or frequency of albuterol extended-release tablets without consulting your physician. If you find that treatment with albuterol extended-release tablets becomes less effective for symptomatic relief, your symptoms become worse, and/or you need to use the product more frequently than usual, you should seek medical attention immediately. While you are using albuterol extended-release tablets, other inhaled drugs and asthma medications should be taken only as directed by your physician. Common adverse effects include palpitations, chest pain, rapid heart rate, tremor or nervousness. If you are pregnant or nursing, contact your physician about use of albuterol extended-release tablets. Effective and safe use of albuterol extended-release tablets includes an understanding of the way that it should be administered.
From 
Lung Disease/COPD Resources

Report Problems to the Food and Drug Administration
You are encouraged to report negative side effects of prescription drugs to the FDA. Visit the FDA MedWatch website or call 1-800-FDA-1088.