
1. Introduction
Diabetes mellitus (DM) has been defined as a chronic disease with persistently elevated blood glucose concentration, leading to acute or long-term complications [1] . Globally, DM presents enormous and increasingly important public health issues. The prevalence of DM in all age groups was estimated to be 2.8% (170 million) in 2000 and the rate is expected to rise to 4.4% (366 million) in 2030 [2] . The pharmacological agents currently used for treatment of diabetes include sulfonylureas, biguanide, thiazolidinedione and glycosidase inhibitors. These agents, however, have restricted usage due to several undesirable side effects and fail to significantly alter the course of diabetic complications [3] . Renewed attention to alternative medicines and natural therapies has stimulated new waves of research interest in traditional practices, and there is a need to look for more efficacious agents with lesser side effects. Presently, there is a growing interest in herbal remedies due to the side effects associated with the oral hypoglycemic agents for the treatment of diabetes mellitus [4] .
Moringa oleifera is a small tree that grows up to 12 feet in height. Its leaves are small and pale-green. It grows in all parts of Nigeria, covering the entire Guinea Savannah zone of the country. Moringa oliefra is a versatile and exceptionally nutritious vegetable tree with a variety of potential uses. It is the most widely cultivated species of a Moringaceae family [5] . Commonly it is known as Moringa or Drumstick tree or Horseradish tree in English, in Hindi as Sahjan, in Latin—Moringa oleifera, in Sanskrit as Surajana, in Nepali Sajiwan or Swejan etc. It is useful not only for human beings but also for animals and also in various industrial applications [6] . The leaves, fruit, flowers and immature pods of this tree are used as a highly nutritive vegetable in many countries, particularly in India, Pakistan, Philippines, Hawaii and many parts of Africa. People in India have been using it as an item of their daily food for nearly 5000 years [7] . It originated initially in the northern part of India some 5000 years ago and soon moved into the southern parts as well, where it was known as “Murungai keerai” (Moringa leaves) and “Murungai kaai” (Moringa vegetable). The Moringa tree had spread to most parts of Asia, nearly the whole of Africa, South America, southern part of North America and some pockets of Europe [8] . It has been found useful in nutrition, agriculture, soil control, water purification, industrial applications, cattle feed etc. and also for treating various types of illnesses in humans and livestock. It is also used as a vegetable and oil source. Moringa pods are an important commercial vegetable crop throughout India. The leaves have a high protein content of 27% and are rich in vitamins A and C, ß carotene, potassium, calcium, iron and phosphorus and act as a good source of natural antioxidants and thus enhance the shelf-life of fat containing foods due to the presence of various types of antioxidant compounds such as ascorbic acid, flavonoids, phenolics and carotenoids [9] .
Moringa oleifera has gained usefulness in pharmacological activities—crude ethanolic extract of dried seeds, hot water infusion of flowers, leaves, roots, seeds and bark, crude methanolic extract of the roots are used as antiinflammatory agents. Oil from dried seeds, methanol and ethanol extract of free dried leaves is also used as antioxidant agents. Defatted and shell-free seeds, fresh leaves juice, roots and bark are antimicrobial [10] ; aqueous extract of stem bark, ethanolic extract of leaves, ethanolic and aqueous extracts of whole pod and their parts, namely, coat, pulp and seed are used as cardiovascular agents [11] . Leaves and fruits are used as antihyperlipidemic agents [12] . Methanolic extract of roots are CNS depressants [13] . Paste of leaves, ethanolic extracts of seeds are used as anticancer agents [14] . Aqueous and ethanolic extract of roots and flower serves as antihepatotoxic agents [15] , Ethanolic extracts and methanolic extracts of leaves and flower buds are use as antiulcer agents [16] . Also, much research has been done to determine the efficacy of this plant; as such the present research designed towards establishing its efficacy in single and in combination with another plant on diabetic and non-diabetic animals.
Peristrophe bicalyculata (Retz.) Nees. (Acanthaceae) is an erect, hispid herb or under shrub of about 60 - 120 cm in height which is found in forest undergrowth, hedges and waste land almost throughout Nigeria. Peristrophe bicalyculata belongs to the Kingdom: plantae, phylum: Magnoliophyta, class Magnoliopsida, order Lamiales, family Acanthaceae and to the genus Peristrophe. It is called “tubanin dawaki” by Hausas in Northern Nigeria, meaning floor of the horse. In the Indore district of India, the local name is “Chotiharjori” [17] . It is native to warm tropical regions of Africa, in the Sahel part of the region of Mauritania, Niger and northern Nigeria as well as in India, Burma and Thailand. The leaves of the plant were used traditionally as analgesic, antipyretic, anti-inflammatory, sedative, stomachic, anticancer, fertility, diuretics and diarrhoea. This plant is used by the traditional healers for curing many skin-related problems; it is also used as an antidote for snake poison when macerated in an infusion of rice, and as an insect repellant. This plant is also used for horse feed and ploughed into the soil as green manure [18] .
Both Moringa olifera and Peristrophe bicalyculata are well-known plants in Nigeria which have found wide use and applications in traditional medicine for the treatment and management of a wide range of disease conditions.
Hyperlipidemia contributes significantly in the manifestation and development of atherosclerosis and coronary heart diseases (CHD). Cardiovascular diseases, including atherosclerosis are the most common cause of mortality and morbidity worldwide [19] . Although several factors, such as diet rich in saturated fats and cholesterol, age, family history, hypertension and life style play a significant role in causing heart failure, the high levels of cholesterol particularly LDL cholesterol are mainly responsible for the onset of CHDs [19] [20] . Lowering lipids and cholesterol levels by a drug or dietary interventions could reduce the risk of CHD. Current interest in natural products has stimulated the search for new cholesterol-lowering agents from these sources. Many herbal medicinal products were reported to have a potential to reduce lipid and cholesterol in body and to enhance the safety profile. Studies have reported that ethanolic extract obtained from the leaves of Moringa oleifera in single and in combination with Occimum gratissimum and Vernonia amygdalina has a significant reduction in serum total cholesterol (Tc), low-density lipoprotein cholesterol (LDL-c), high-density lipoprotein cholesterol (HDL-c) and triglycerides (TG) [21] with no report on Peristrophe bicalyculata—a traditionally acclaim medicinal plant used in the management of cardiovascular-related complications. This study was dedicated to monitoring changes in the lipid profile of male albino rats under the anti-lipidemic effects of combined extract of Moringa oleifera and Peristrophe bicalyculata.
2. Materials and Methods
2.1. Collection of Plant Materials
Fresh leaves of Peristrophe bicalyculata was harvested from the Endocrine Research Farm while Moringa olifera was harvested from the staff village environment, University of Calabar. They were authenticated by a botanist Dr Mike Eko, Department of Botany, University of Calabar, Calabar and Voucher specimens number ERU/2011/345 and specimens deposited in a herbarium in the Department of Botany.
The leaves were rinsed severally with clean tap water to remove dust particles and debris followed with distilled water thereafter allowed to completely drain. The dry Peristrophe bicalyculata and Moringa olifera leaves were blended with the use of cornono (El legitima) V C.I.A. S. A manual hand blended, made in Colombia into powdered and 3 kg of of Peristrophe bicalyculata was weighed out and soak with in 2000 ml of 80% ethanol while 800 g of Moringa olifera weighed out and soaked in 1000 ml of 80% ethanol. The mixtures were allowed for 48 hours in the refrigerator at 4˚C for thorough extraction of the plants’ active components. These were then filtered with a chess material and later with Whatman No. 1 filter paper to obtain a homogenous filtrate. These filtrates were then concentrated in vacuo at low temperature (37˚C - 40˚C) to about one tenth the original volume using a rotary evaporator. The concentrates were allowed open in a water bath (40˚C) for complete dryness yielding 34.9 g (3.49%) and 29 g (3.62%) respectively. The extracts were then refrigerated at 2˚C - 8˚C until when used.
2.2. Animals
Fifty four albino rats (males only) of Wistar strain weighing about 140 - 180 g were obtained from the animal house of the Department of Biochemistry, University of Calabar, Calabar. The animals were allowed to acclimatize for a weeks in the animal house of the department of biochemistry. The animals were housed in well ventilated cages (wooden bottom and wire mesh top) and kept under controlled environmental conditions of temperature (25˚C ± 5˚C), relative humidity (50% ± 5%) and 12 hour light/dark cycle. The protocol was in accordance with the guidelines of the National Institute of Health (NIH) publication (1985) for laboratory Animal Care and Use and approved by the Collage of Medical Sciences Animal Ethics Committee University of Calabar, Nigeria.
2.3. Experimental Design
The design consisted of 54 rats divided into 5 parallel groups consisting of a diabetic and non-diabetic pair of 6 animals each (Table 1). The doses used were based on the predetermined LD50 values obtained from preliminary studies.
2.4. Acute Toxicity Test
The LD50 for the ethanolic extract was determined using the method described by [22] .
2.5. Induction of Experimental Diabetes
Hyperglycemia was induced by a single i.p. injection of 100 mg/kg of alloxan monohydrate (Sigma-Aldrich, Inc., St. Louis, MO 63103, USA) in sterile saline. After 5 days of alloxan injection, the hyperglycemic rats (glucose level > 150 mg/dl) were separated and divided into different groups comprising of 6 rats each for the anti-diabetic study.
2.6. Extract and Drug Administration
Before administration, the extracts were reconstituted in normal saline (vehicle) and administered orally via gastric intubation at a dose of 500 mg/kg b.w for the single dose and 250 mg/kg for the combine extract treatment. Insulin was administered at 5 IU/kg b.w. The control animal received 0.2 ml of normal saline (placebo).
2.7. Experimental Procedure
Diabetic and non-diabetic animals were grouped as shown in Table 1. The dosages of the plant extracts were as determined from preliminary work in our laboratory whereas insulin dose, NPH (5 U/kg b.w. s.c.)
The plant extracts was administered via oral gastric intubation, twice per day (10.00 am: 4.00 pm) in a 6 hrs cycle and insulin once per day post prandial (10.00 am), subcutaneously (s.c.). Treatment lasted for 14 days and throughout this periodic changes in blood glucose and body weight were measured with the use of a glucometer and animal weighing balance respectively. The animals were maintained on pellets prepared with Growers feed from Vital Feeds, Jos, Plateau state, Nigeria, and tap water. Both the feed and water were provided ad libitum.
2.8. Collection of Samples for Analysis
At the end of the 14 days, food was withdrawn from the rats and they were fasted overnight but had free access to water. They were then euthanized under chloroform vapor and sacrificed. Whole blood was collected via car

Table 1 . Animal grouping and treatment schedule.
diac puncture using sterile syringes and needles. The blood was emptied into plain tubes and allowed to clot for about two hours. The clotted blood was thereafter centrifuged at 3000 rpm for 10 minutes to recover serum from clotted cells. Serum was separated with sterile syringes and needles and stored frozen until used for biochemical analysis.
2.9. Estimation of Serum Lipid Profile Level
Serum lipid profile viz triacylglycerol (TG), total cholesterol (TC), low density lipoprotein cholesterol (LDL), High density lipoprotein cholesterol (HDL-C) were estimated colorimetrically using assay kit method from AGAPPE diagnostic (Switzerland) according to manufactured instruction.
2.10. Data and Statistical Analysis
The results were analyzed for statistical significance by one way Anova (Analysis of variance) with a post hoc Dunnet at p value < 0.05 t using SPSS software. All data were expressed as mean ± SEM (n = 6 replications).
3. Result
3.1. Effect of Extracts on Lipid Profile
Effect of administration of extracts of Moringa oleifera and Peristrophe bicalyculata on serum lipid profile viz: Triacylglycerol (TG), Total cholesterol (TC), High density lipoprotein-cholesterol (HDL-C), Very low density lipoprotein (VLDL) and low density lipoprotein (LDL) in experimental rat models after 14 days experimental period was investigated and results obtained are shown in Table 2. From the result, significant increase (p < 0.05) was observed in TG concentration of all treated groups compared to the diabetic control group. TC level (mg/dl) was significantly increased (p < 0.05) for all the treated groups when compared to the normal control group. Also, no significant change (p > 0.05) was observed in HDL-C level in treated groups when compared to the normal control, diabetic control and insulin groups. Concentration of VLDL was significantly decreased (p < 0.05) in the diabetic control group and insulin group when compared to the normal control group, on treatment, a significant increase (p < 0.05) was observed in the VLDL concentration of all the treated groups compared to the diabetic control and insulin groups. LDL concentration (mg/dl) was significantly increased (p < 0.05) in all treated groups when compared to the normal control group.
3.2. Changes in Body Weight
Figure 1 showed a comparison changes in the daily body weight of different experimental group treated with extracts and insulin respectively. From the result, a slight decrease in body weight of the diabetic treated and

Table 2. Effect of extracts of Moringa oliefera and Peristrophe bicalyculata on lipid profile of experimental rat models.
*p < 0.05 vs NC; a = p < 0.05 vs DC; b = p < 0.05 vs in DC = Diabetic control, NC = Normal control, NPB + NMO = Normal Peristrphe bicaliculata + Moringa oliefera, DPB = Diabetic Peristrphe bicaliculata, DMO = Diabetic Moringa oliefera, DPB + DMO = Peristrphe bicaliculata + Moringa oliefera. Values are expressed as mean ± SEM. n = 6.

Figure 1. Comparison of the daily body weights of the different experimental groups. Values are mean ± SEM, n = 6.
diabetic control group was observed at day 2 of the experimental period compared to normal. However, this decrease in body weight of diabetic treated and diabetic control was consistent for day 5, 6, 7th with peak increase at the 8th day and further decreases at the end of the experimental period. Also observed from the result was a comparison of the initial and final body weight within the experimental period Figure 2, increase in body weight of all experimental treated groups was observed with no significant (p > 0.05) comparison. In all, increase in body weight change was observed in diabetic control and insulin group Figure 3 compared to the normal control and extract treated groups, although not significant (p > 0.05).
4. Discussion
The traditional system of medicinal plants and practices have been an imperative resource in many countries to control various complications of diabetes mellitus as they are considered to be less toxic and free from side effects than synthetic molecules. Moringa oleifera and Peristrophr bicalyculata are among the important plants with potent hypolipidemic effect [23] as choice of alternative medicine for treating diabetes and it related complications. Coronary heart disease (CHD) is a major cause of morbidity and mortality worldwide. Epidemiological, genetic, laboratory animal and metabolic ward studies have provided evidence linking high blood cholesterol with atherosclerosis and CHD [23] [24] . While cholesterol is believed to be chiefly implicated in the relationship, serum triacylglycerols may also play a role [25] . Elevated low density lipoprotein cholesterol (LDL-C) and decreased high density lipoprotein cholesterol (HDL-C) levels are well recognized CHD risk factors with recent evidence supporting the benefit of intensive LDL-C reduction on CHD risk [26] . High levels of HDL are negatively associated with the risk of CHD; high levels of TG, which in the fasting condition are found mainly in VLDL, are positively related to the risk for CHD [27] . As LDL carries most of the plasma cholesterol, the total plasma cholesterol may also be a good index for the risk of CHD, when the high cholesterol level is not due to a high HDL level. However the total cholesterol to HDL ratio may be the most efficient predictor for the risk of CHD [28] . Results of lipids assayed in this investigation showed that there was significant decreased and increase (p < 0.05) in TG, TC, VLDL and LDL-C in all treated groups compared to diabetic and normal control groups. HDL-C was not significantly different in all treated groups.
It is an established fact that growth in all life forms results from a surplus in energy balance between intake and expenditure. This usually is compromised in pathological conditions where more often food is barely tolerated and the entire metabolism of the individual is adversely affected. More so, a metabolic impairment leading to imbalance in energy can also result from impressed factors including drastic changes in environmental conditions, exposure to drugs, toxicants, pollutants and the likes [29] . Studies have shown an association between
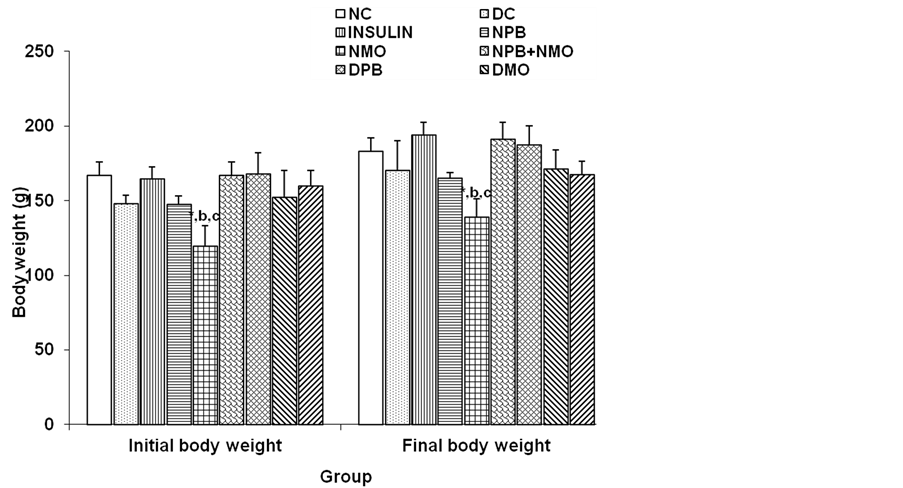
Figure 2. Comparison of the initial and final body weights of the different experimental groups. Values are mean ± SEM, n = 6. *p < 0.05 vs NC; b = p < 0.05 vs insulin; c = p < 0.05 vs NPB + NMP.

Figure 3. Comparison of the body weight change of the different experimental groups. Values are mean ± SEM, n = 6. b = p < 0.05 vs insulin.
hyperglycemia and decrease in body weight of experimental animals [30] . Consequently, changes in weight are usually a fundamental index of physiological or pathological state of an experimental animal. From this study, the sharp decrease in body weight of diabetic control compared to normal control and experimental treated animal during experimental period is an indication in tissue wasting as result of poor glycemic control in diabetes mellitus and this usually foster protein and fat mobilization [31] . However, weight again in the other hand was observed in the diabetic groups treated with the extract and insulin respectively. This increase in body weight was compromise in groups that were co-administered with the two plant extracts. This could be due to a better control of hyperglycemic state in the diabetic rats and decreased fasting blood glucose level could improve body weight in alloxan-induced diabetic rats [32] .
5. Conclusion
In conclusion, this study demonstrated that the combined leaf extracts of Moringa oleifera and Peristrophe bicalyculata beneficiated the management of non-communicable diseases such as hyperglycemia and hyperlipidemia and that some phytochemicals in the two plants are implicated in the reversal of progressive diabetes and its complications.
Abbreviations
INS: Insulin LDL-C: Low Density Lipoprotein: Cholesterol MO: Moringa oleifera NC: Normal Control DC: Diabetic Control TC: Total Cholesterol TG: Triacylglyceride VLDL: C-Very Low Density Lipoprotein-Cholesterol NPB + NMO: Normal Peristrphe bicaliculata + Moringa oliefera DPB: Diabetic Peristrphe bicaliculata DMO = Diabetic Moringa oliefera DPB + DMO: Peristrphe bicaliculata + Moringa oliefera
NOTES
*Corresponding author.