- Uses
- Warnings
- Side Effects
- Dosage
- Addiction/Overdose
- Drug Interactions
- Pregnancy & Breastfeeding
- What Else to Know
-
Comments
-
**COMMENTSTAGLIST**
-
More
-
**OTHERTAGLIST**
Brand Name: Paxlovid
Generic Name: nirmatrelvir; ritonavir
Drug Class: Antivirals
What is Paxlovid, and what is it used for?
Paxlovid is a prescription medicine used to treat the symptoms of COVID-19. Paxlovid may be used alone or with other medications.
Paxlovid belongs to a class of drugs called Antivirals, SARS-CoV-2.
It is not known if Paxlovid is safe and effective in children younger than 12 years of age or weighing less than 88 pounds (40 kg).
Warnings
The U.S. Food and Drug Administration (FDA) has issued an Emergency Use Authorization (EUA) for the emergency use of the unapproved product Paxlovid for the treatment of mild-to-moderate coronavirus disease 2019 (COVID-19) in adults and pediatric patients (12 years of age and older weighing at least 40 kg) with positive results of direct severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) viral testing, and who is at high risk for progression to severe COVID-19, including hospitalization or death.
For information on medical conditions and factors associated with increased risk for progression to severe COVID-19, see the Centers for Disease Control and Prevention (CDC) website: https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/people-with-medical-conditions.html. Healthcare providers should consider the benefit-risk for an individual patient.
Limitations Of Authorized Use
- Paxlovid is not authorized for initiation of treatment in patients requiring hospitalization due to severe or critical COVID-19.
- Paxlovid is not authorized for use as pre-exposure or post-exposure prophylaxis for prevention of COVID-19.
- Paxlovid is not authorized for use for longer than 5 consecutive days.
Paxlovid may only be prescribed for an individual patient by physicians, advanced practice registered nurses, and physician assistants that are licensed or authorized under state law to prescribe drugs in the therapeutic class to which Paxlovid belongs (i.e., antiinfectives).
Paxlovid is not approved for any use, including for use for the treatment of COVID-19.
Paxlovid is authorized only for the duration of the declaration that circumstances exist justifying the authorization of the emergency use of Paxlovid under section 564(b)(1) of the Act, 21 U.S.C. § 360bbb-3(b)(1), unless the authorization is terminated or revoked sooner.
Patients requiring hospitalization due to severe or critical COVID-19 after starting treatment with Paxlovid may complete the full 5-day treatment course per the healthcare provider's discretion.
Justification For Emergency Use Of Drugs During The COVID-19 Pandemic
There is currently an outbreak of COVID-19 caused by SARS-CoV-2, a novel coronavirus. The Secretary of Health and Human Services (HHS) has declared that:
- A public health emergency related to COVID-19 has existed since January 27, 2020.
- Circumstances exist justifying the authorization of emergency use of drugs and biological products during the COVID-19 pandemic (March 27, 2020 declaration).
An EUA is a U.S. Food and Drug Administration authorization for the emergency use of an unapproved product or unapproved use of an approved product (i.e., drug, biological product, or device) in the United States under certain circumstances including, but not limited to, when the Secretary of HHS declares that there is a public health emergency that affects the national security or the health and security of United States citizens living abroad, and that involves biological agent(s) or a disease or condition that may be attributable to such agent(s). Criteria for issuing an EUA include:
- The biological agent(s) can cause a serious or life-threatening disease or condition;
- Based on the totality of the available scientific evidence (including data from adequate and well-controlled clinical trials, if available), it is reasonable to believe that
- the product may be effective in diagnosing, treating, or preventing the serious or life-threatening disease or condition; and
- the known and potential benefits of the product—when used to diagnose, prevent, or treat such disease or condition—outweigh the known and potential risks of the product, taking into consideration the material threat posed by the biological agent(s);
- There is no adequate, approved, and available alternative to the product for diagnosing, preventing, or treating the serious or life-threatening disease or condition.
Information Regarding Available Alternatives for The EUA Authorized Use
- There are no approved alternatives to Paxlovid for the treatment of mild-to-moderate COVID-19 in adults and pediatric patients (12 years of age and older weighing at least 40 kg) with positive results of direct SARS-CoV-2 viral testing, and who are at high risk for progression to severe COVID-19, including hospitalization or death.
- Other therapeutics are currently authorized for the same use as Paxlovid. For additional information on all products authorized for treatment or prevention of COVID-19, please see https://www.fda.gov/emergency-preparedness-and-response/mcm-legal-regulatory-and-policy- framework/emergency-use-authorization.
- For information on clinical studies that are testing the use of Paxlovid in COVID-19, please see https:www.clinicaltrials.gov.
What are the side effects of Paxlovid?
There are limited clinical data available for Paxlovid. Serious and unexpected adverse events may occur that have not been previously reported with Paxlovid use.
Paxlovid may cause serious side effects including:
- hives,
- difficulty breathing,
- swelling of your face, lips, tongue, or throat,
- severe dizziness, and
- abnormal lab test results
Get medical help right away, if you have any of the symptoms listed above.
The most common side effects of Paxlovid include:
- diarrhea,
- myalgia,
- altered sense of taste, and
- high blood pressure
Tell the doctor if you have any side effect that bothers you or that does not go away.
These are not all the possible side effects of Paxlovid. For more information, ask your doctor or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
What are the dosages of Paxlovid?
Dosage for Emergency Use of Paxlovid
- Paxlovid is nirmatrelvir tablets co-packaged with ritonavir tablets.
- Nirmatrelvir must be co-administered with ritonavir. Failure to correctly co-administer nirmatrelvir with ritonavir may result in plasma levels of nirmatrelvir that are insufficient to achieve the desired therapeutic effect.
- The dosage for Paxlovid is 300 mg nirmatrelvir (two 150 mg tablets) with 100 mg ritonavir (one 100 mg tablet) with all three tablets taken together orally twice daily for 5 days. Prescriptions should specify the numeric dose of each active ingredient within Paxlovid. Completion of the full 5-day treatment course and continued isolation in accordance with public health recommendations are important to maximize viral clearance and minimize transmission of SARS-CoV-2.
- The 5-day treatment course of Paxlovid should be initiated as soon as possible after a diagnosis of COVID-19 has been made, and within 5 days of symptom onset. Should a patient require hospitalization due to severe or critical COVID-19 after starting treatment with Paxlovid, the patient should complete the full 5-day treatment course per the healthcare provider's discretion.
- If the patient misses a dose of Paxlovid within 8 hours of the time it is usually taken, the patient should take it as soon as possible and resume the normal dosing schedule. If the patient misses a dose by more than 8 hours, the patient should not take the missed dose and instead take the next dose at the regularly scheduled time. The patient should not double the dose to make up for a missed dose.
- Paxlovid (both nirmatrelvir and ritonavir tablets) can be taken with or without food. The tablets should be swallowed whole and not chewed, broken, or crushed.
Important Dosing Information in Patients with Renal Impairment
- No dosage adjustment is needed in patients with mild renal impairment (eGFR =60 to <90 mL/min). In patients with moderate renal impairment (eGFR =30 to <60 mL/min), the dosage of Paxlovid is 150 mg nirmatrelvir and 100 mg ritonavir twice daily for 5 days. Prescriptions should specify the numeric dose of each active ingredient within Paxlovid. Providers should counsel patients about renal dosing instructions.
- Paxlovid is not recommended in patients with severe renal impairment (eGFR <30 mL/min) until more data are available; the appropriate dosage for patients with severe renal impairment has not been determined.
Use In Patients with Hepatic Impairment
- No dosage adjustment is needed in patients with mild (Child-Pugh Class A) or moderate (Child-Pugh Class B) hepatic impairment. No pharmacokinetic or safety data are available regarding the use of nirmatrelvir or ritonavir in subjects with severe hepatic impairment (Child-Pugh Class C); therefore, Paxlovid is not recommended for use in patients with severe hepatic impairment.
Important Drug Interactions with Paxlovid
- No dosage adjustment is required when co-administered with other products containing ritonavir or cobicistat.
- Patients on ritonavir- or cobicistat-containing HIV or HCV regimens should continue their treatment as indicated.
- Refer to other sections of the Fact Sheet for important drug interactions with Paxlovid. Consider the potential for drug interactions prior to and during Paxlovid therapy and review concomitant medications during Paxlovid therapy.
Addiction/overdose
Treatment of overdose with Paxlovid should consist of general supportive measures including monitoring of vital signs and observation of the clinical status of the patient. There is no specific antidote for overdose with Paxlovid.
What drugs interact with Paxlovid?
Potential for Paxlovid To Affect Other Drugs
- Paxlovid (nirmatrelvir co-packaged with ritonavir) is an inhibitor of CYP3A and may increase plasma concentrations of drugs that are primarily metabolized by CYP3A.
- Co-administration of Paxlovid with drugs highly dependent on CYP3A for clearance and for which elevated plasma concentrations are associated with serious and/or life-threatening events is contraindicated. Co-administration with other CYP3A substrates may require a dose adjustment or additional monitoring as shown in Table 1.
Potential for Other Drugs to Affect Paxlovid
- Nirmatrelvir and ritonavir are CYP3A substrates; therefore, drugs that induce CYP3A may decrease nirmatrelvir and ritonavir plasma concentrations and reduce Paxlovid therapeutic effect.
Established and Other Potentially Significant Drug Interactions
- Table 1 provides a listing of clinically significant drug interactions, including contraindicated drugs. Drugs listed in Table 1 are a guide and not considered a comprehensive list of all possible drugs that may interact with Paxlovid. The healthcare provider should consult appropriate references for comprehensive information.
Table 1: Established and Other Potentially Significant Drug Interactions
| Drug Class | Drugs within Class | Effect on Concentration | Clinical Comments |
| Alpha 1-adrenoreceptor antagonist | alfuzosin | ↑ alfuzosin | Co-administration contraindicated due to potential hypotension. |
| Analgesics | pethidine, piroxicam, propoxyphene |
↑ pethidine ↑ piroxicam ↑ propoxyphene |
Co-administration contraindicated due to potential for serious respiratory depression or hematologic abnormalities. |
| Antianginal | ranolazine | ↑ ranolazine | Co-administration contraindicated due to potential for serious and/or life-threatening reactions. |
| Antiarrhythmics | amiodarone, dronedarone, flecainide, propafenone, quinidine |
↑ antiarrhythmic | Co-administration contraindicated due to potential for cardiac arrhythmias. |
| Antiarrhythmics | bepridil, lidocaine (systemic) | ↑ antiarrhythmic | Caution is warranted and therapeutic concentration monitoring is recommended for antiarrhythmics if available. |
| Anticancer drugs | apalutamide | ↓ nirmatrelvir/ritonavir | Co-administration contraindicated due to potential loss of virologic response and possible resistance. |
| Anticancer drugs | abemaciclib, ceritinib, dasatinib, encorafenib, ibrutinib, ivosidenib, neratinib, nilotinib, venetoclax, vinblastine, vincristine |
↑ anticancer drug | Avoid co-administration of encorafenib or ivosidenib due to potential risk of serious adverse events such as QT interval prolongation. Avoid use of neratinib, venetoclax or ibrutinib. Co-administration of vincristine and vinblastine may lead to significant hematologic or gastrointestinal side effects. For further information, refer to individual product label for anticancer drug. |
| Anticoagulants | warfarin rivaroxaban |
↑↓ warfarin ↑ rivaroxaban |
Closely monitor INR if co-administration with warfarin is necessary. Increased bleeding risk with rivaroxaban. Avoid concomitant use. |
| Anticonvulsants | carbamazepine* , phenobarbital, phenytoin |
↓ nirmatrelvir/ritonavir ↑ carbamazepine ↓ phenobarbital ↓ phenytoin |
Co-administration contraindicated due to potential loss of virologic response and possible resistance. |
| Antidepressants | bupropion | ↓ bupropion and active metabolite hydroxybupropion | Monitor for an adequate clinical response to bupropion. |
| trazodone | ↑ trazodone | Adverse reactions of nausea, dizziness, hypotension, and syncope have been observed following co-administration of trazodone and ritonavir. A lower dose of trazodone should be considered. Refer to trazadone product label for further information. | |
| Antifungals | voriconazole, ketoconazole, isavuconazonium sulfate itraconazole* |
↓ voriconazole ↑ ketoconazole ↑ isavuconazonium sulfate ↑ itraconazole ↑ nirmatrelvir/ritonavir |
Avoid concomitant use of voriconazole. Refer to ketoconazole, isavuconazonium sulfate, and itraconazole product labels for further information. |
| Anti-gout | colchicine | ↑ colchicine | Co-administration contraindicated due to potential for serious and/or life-threatening reactions in patients with renal and/or hepatic impairment. |
| Anti-HIV protease inhibitors | amprenavir, atazanavir, darunavir, fosamprenavir, indinavir, nelfinavir, saquinavir, tipranavir |
↑ protease Inhibitor | For further information, refer to the respective protease inhibitors' prescribing information. Patients on ritonavir- or cobicistatcontaining HIV regimens should continue their treatment as indicated. Monitor for increased Paxlovid or protease inhibitor adverse events with concomitant use of these protease inhibitors. |
| Anti-HIV | didanosine, delavirdine, efavirenz, maraviroc, nevirapine, raltegravir, zidovudine bictegravir/ emtricitabine/ tenofovir |
↑ didanosine ↑ efavirenz ↑ maraviroc ↓ raltegravir ↓ zidovudine ↑ bictegravir ↔ emtricitabine ↑ tenofovir |
For further information, refer to the respective anti-HIV drugs prescribing information. |
| Anti-infective | clarithromycin, erythromycin |
↑ clarithromycin ↑ erythromycin |
Refer to the respective prescribing information for anti-infective dose adjustment. |
| Antimycobacterial | rifampin | ↓ nirmatrelvir/ritonavir | Co-administration contraindicated due to potential loss of virologic response and possible resistance. Alternate antimycobacterial drugs such as rifabutin should be considered. |
| Antimycobacterial | bedaquiline rifabutin |
↑ bedaquiline ↑ rifabutin |
Refer to the bedaquiline product label for further information. Refer to rifabutin product label for further information on rifabutin dose reduction. |
| Antipsychotics | lurasidone, pimozide, clozapine |
↑ lurasidone ↑ pimozide ↑ clozapine |
Co-administration contraindicated due to serious and/or life-threatening reactions such as cardiac arrhythmias. |
| Antipsychotics | quetiapine | ↑ quetiapine | If co-administration is necessary, reduce quetiapine dose and monitor for quetiapine-associated adverse reactions. Refer to the quetiapine prescribing information for recommendations. |
| Calcium channel blockers | amlodipine, diltiazem, felodipine, nicardipine, nifedipine |
↑ calcium channel blocker | Caution is warranted and clinical monitoring of patients is recommended. A dose decrease may be needed for these drugs when co-administered with Paxlovid. If co-administered, refer to individual product label for calcium channel blocker for further information. |
| Cardiac glycosides | digoxin | ↑ digoxin | Caution should be exercised when coadministering Paxlovid with digoxin, with appropriate monitoring of serum digoxin levels. Refer to the digoxin product label for further information. |
| Endothelin receptor Antagonists | bosentan | ↑ bosentan | Discontinue use of bosentan at least 36 hours prior to initiation of Paxlovid. Refer to the bosentan product label for further information. |
| Ergot derivatives | dihydroergotamine, ergotamine, methylergonovine |
↑ dihydroergotamine ↑ ergotamine ↑ methylergonovine |
Co-administration contraindicated due to potential for acute ergot toxicity characterized by vasospasm and ischemia of the extremities and other tissues including the central nervous system. |
| Hepatitis C direct acting antivirals | elbasvir/grazoprevir, glecaprevir/pibrentasvir | ↑ antiviral | Increased grazoprevir concentrations can result in ALT elevations. It is not recommended to co-administer ritonavir with glecaprevir/pibrentasvir. Refer to the ombitasvir/paritaprevir/ritonavir and dasabuvir label for further information. Refer to the sofosbuvir/velpatasvir/voxilaprevir product label for further information. Patients on ritonavir-containing HCV regimens should continue their treatment as indicated. Monitor for increased Paxlovid or HCV drug adverse events with concomitant use. |
| ombitasvir/paritaprevir/ ritonavir and dasabuvir |
|||
| sofosbuvir/velpatasvir/ voxilaprevir |
|||
| Herbal products | St. John's Wort (hypericum perforatum) | ↓ nirmatrelvir/ritonavir | Co-administration contraindicated due to potential loss of virologic response and possible resistance. |
| HMG-CoA reductase inhibitors | lovastatin, simvastatin |
↑ lovastatin ↑ simvastatin |
Co-administration contraindicated due to potential for myopathy including rhabdomyolysis. Discontinue use of lovastatin and simvastatin at least 12 hours prior to initiation of Paxlovid. |
| HMG-CoA reductase inhibitors | atorvastatin, rosuvastatin |
↑ atorvastatin ↑ rosuvastatin |
Consider temporary discontinuation of atorvastatin and rosuvastatin during treatment with Paxlovid. |
| Hormonal contraceptive | ethinyl estradiol | ↓ ethinyl estradiol | An additional, non-hormonal method of contraception should be considered. |
| Immunosuppressants | cyclosporine, tacrolimus, sirolimus |
↑ cyclosporine ↑ tacrolimus ↑ sirolimus |
Therapeutic concentration monitoring is recommended for immunosuppressants. Avoid use of Paxlovid when close monitoring of immunosuppressant serum concentrations is not feasible. Avoid concomitant use of sirolimus and Paxlovid. If co-administered, refer to individual product label for immunosuppressant for further information. |
| Long-acting betaadrenoceptor agonist | salmeterol | ↑ salmeterol | Co-administration is not recommended. The combination may result in increased risk of cardiovascular adverse events associated with salmeterol, including QT prolongation, palpitations, and sinus tachycardia. |
| Narcotic analgesics | fentanyl | ↑ fentanyl | Careful monitoring of therapeutic and adverse effects (including potentially fatal respiratory depression) is recommended when fentanyl is concomitantly administered with Paxlovid. |
| methadone | ↓ methadone | Monitor methadone-maintained patients closely for evidence of withdrawal effects and adjust the methadone dose accordingly. | |
| PDE5 inhibitor | sildenafil (Revatio®) when used for pulmonary arterial hypertension | ↑ sildenafil | Co-administration contraindicated due to the potential for sildenafil associated adverse events, including visual abnormalities hypotension, prolonged erection, and syncope. |
| Sedative/hypnotics | triazolam, oral midazolam |
↑ triazolam ↑ midazolam |
Co-administration contraindicated due to potential for extreme sedation and respiratory depression. |
| Sedative/hypnotics | midazolam (administered parenterally) | ↑ midazolam | Co-administration of midazolam (parenteral) should be done in a setting which ensures close clinical monitoring and appropriate medical management in case of respiratory depression and/or prolonged sedation. Dosage reduction for midazolam should be considered, especially if more than a single dose of midazolam is administered. Refer to the midazolam product label for further information. |
| Systemic corticosteroids | betamethasone, budesonide, ciclesonide, dexamethasone, fluticasone, methylprednisolone, mometasone, prednisone, triamcinolone |
↑ corticosteroid | Increased risk for Cushing's syndrome and adrenal suppression. Alternative corticosteroids including beclomethasone and prednisolone should be considered. |
Pregnancy and breastfeeding
- There are no available human data on the use of nirmatrelvir during pregnancy to evaluate for a drug-associated risk of major birth defects, miscarriage, or adverse maternal or fetal outcomes.
- Published observational studies on ritonavir use in pregnant women have not identified an increase in the risk of major birth defects. Published studies with ritonavir are insufficient to identify a drug-associated risk of miscarriage. There are maternal and fetal risks associated with untreated COVID-19 in pregnancy.
- There are no available data on the presence of nirmatrelvir in human or animal milk, the effects on the breastfed infant, or the effects on milk production. There is no information on the effects of ritonavir on the breastfed infant or the effects of the drug on milk production.
- The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for Paxlovid and any potential adverse effects on the breastfed infant from Paxlovid or from the underlying maternal condition.
- Breastfeeding individuals with COVID-19 should follow practices according to clinical guidelines to avoid exposing the infant to COVID-19.
What else should I know about Paxlovid?
Tell your healthcare provider if you:
- Have any allergies
- Have liver or kidney disease
- Are pregnant or plan to become pregnant
- Are breastfeeding a child
- Have any serious illnesses
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
Some medicines may interact with Paxlovid and may cause serious side effects. Keep a list of your medicines to show your healthcare provider and pharmacist when you get a new medicine.
You can ask your healthcare provider or pharmacist for a list of medicines that interact with Paxlovid. Do not start taking a new medicine without telling your healthcare provider. Your healthcare provider can tell you if it is safe to take Paxlovid with other medicines.
Tell your healthcare provider if you are taking combined hormonal contraceptive. Paxlovid may affect how your birth control pills work. Females who are able to become pregnant should use another effective alternative form of contraception or an additional barrier method of contraception. Talk to your healthcare provider if you have any questions about contraceptive methods that might be right for you.
Do not take Paxlovid if:
- You are allergic to nirmatrelvir, ritonavir, or any of the ingredients in Paxlovid.
- You are taking any of the following medicines:
- Alfuzosin
- Pethidine, piroxicam, propoxyphene
- Ranolazine
- Amiodarone, dronedarone, flecainide, propafenone, quinidine
- Colchicine
- Lurasidone, pimozide, clozapine
- Dihydroergotamine, ergotamine, methylergonovine
- Lovastatin, simvastatin
- Sildenafil (Revatio®) for pulmonary arterial hypertension (PAH)
- Triazolam, oral midazolam
- Apalutamide
- Carbamazepine, phenobarbital, phenytoin
- Rifampin
- St. John's Wort (hypericum perforatum)
Taking Paxlovid with these medicines may cause serious or life-threatening side effects or affect how Paxlovid works.
These are not the only medicines that may cause serious side effects if taken with Paxlovid. Paxlovid may increase or decrease the levels of multiple other medicines. It is very important to tell your healthcare provider about all of the medicines you are taking because additional laboratory tests or changes in the dose of your other medicines may be necessary while you are taking Paxlovid.
Your healthcare provider may also tell you about specific symptoms to watch out for that may indicate that you need to stop or decrease the dose of some of your other medicines.
Summary
Paxlovid is a prescription antiviral medicine used to treat the symptoms of COVID-19 with emergency use authorization (EUA). The most common side effects of Paxlovid include diarrhea, myalgia, altered sense of taste, and high blood pressure. Serious side effects of Paxlovid include hives, difficulty breathing, swelling of your face, lips, tongue, or throat; severe dizziness, and abnormal lab test results. Consult your doctor if pregnant or breastfeeding.
Multimedia: Slideshows, Images & Quizzes
-

Rosacea, Acne, Shingles, Covid-19 Rashes: Common Adult Skin Diseases
Learn to spot and treat skin conditions commonly found in adults such as acne, Covid-19 rashes, eczema, shingles, psoriasis,...
-

Coronavirus: How COVID-19 Affects Your Body
By now, everyone knows about COVID-19. But do you know how it can affect your body?
-

Lung and Respiratory: Signs That You May Have Had COVID-19
Could you have already had COVID-19 and not know it? Learn some signs that might indicate just that.
-

COVID-19 Vaccine Myths and Facts Quiz
The FDA has granted Emergency Use Authorizations for COVID-19 vaccines that have been shown to be safe and effective as...
-

Novel Coronavirus (COVID-19) Prevention Quiz
Why is coronavirus considered dangerous? What are the symptoms you should look for? Take this COVID-19 prevention quiz to learn...
-

Coronavirus COVID-19 Prevention: Test Your Medical IQ
What's really the best way to prevent the spread of new coronavirus COVID-19? Should wear a mask or not? Take this quiz to find...
-

COVID-19 Coronavirus Disease: Articles of Interest
Read about COVID-19 Coronavirus Disease. See interesting articles related to vitamins and supplements to boost immunity,...
-

COVID-19 (Coronavirus): Mask Mistakes You're Making Now
You're wearing your mask every time you leave home. But could you be making simple mistakes that make your mask less effective?...
-

Lung Disease & Respiratory Health: Should I Get a COVID-19 Antibody Test?
If you had COVID-19 symptoms but never got tested, or if you have long-term symptoms that just won't go away, you may want to get...
-

Coronavirus COVID-19 (SARS-CoV-2) Pandemic Outbreak: What You Need to Know
A new strain of coronavirus (COVID-19, SARS-CoV-2) was reported from Wuhan, China in December, 2019. This outbreak of respiratory...
-

How Can Parents Help Children Stay Social During COVID-19?
The COVID-19 pandemic has affected children with fewer social opportunities. As a parent, here's how you can help them socialize...
-

COVID-19: Don't Delay Doctor Visits Because of COVID
You want to protect yourself from COVID-19, but you still need medical care. Find out when it's important to see a doctor.
-

What Drugs May Fight COVID-19? Drug Trials, Treatments, Vaccines
What drugs could help fight coronavirus COVID-19? Clinical studies are ongoing for antiviral drugs like hydroxychloroquine,...
Related Disease Conditions
-

When Do You Lose Your Sense of Smell and Taste With COVID-19?
According to recent studies, COVID-19 symptoms of loss of smell and taste typically begin 4-5 days after other symptoms have appeared and may last 7-14 days.
-

How Do I Stop a COVID-19 Cough?
Cough is a symptom of COVID-19 that can linger for weeks even after the infection has cleared up. Here are 9 tips for stopping a COVID-19 cough.
-

What Do COVID-19 Body Aches Feel Like?
COVID-19 body aches feel like dull muscle pain and can affect the shoulders, lower back, or legs. Learn more about coronavirus symptoms. Check out the center below for more medical references on the coronavirus, including multimedia (slideshows, images, and quizzes), related diseases, treatment, diagnosis, medications, and prevention or wellness.
-
When Does a COVID-19 Patient Need to Go on a Ventilator?
When COVID-19 leads to ARDS, a ventilator is needed to help the patient breathe. ARDS reduces the ability of the lungs to provide enough oxygen to vital organs.
-

COVID-19 (Coronavirus, 2019-nCoV)
Infection with COVID-19 (2019 novel coronavirus, 2019-nCoV) causes respiratory problems in humans. Transmission of COVID-19 occurs mainly through contact with respiratory sections from an infected person, however, fecal contamination may also spread the virus. Symptoms start off flu-like and progress to coughing, fever, shortness of breath, shaking chills, headache, loss of sense of taste and/or smell, muscle pain, and sore throat. Treatment focuses on supportive care and symptom relief. COVID-19 vaccines are available.
-

Does COVID-19 Cause Dizzy Spells?
Although not a typical symptom of COVID-19, neurological symptoms, such as dizziness, are associated with coronavirus infection.
-
Is Your Immune System Stronger After COVID-19?
A robust immune system protects you from getting sick following exposure to germs and viruses. Yes, recovering from COVID-19 makes your immune system stronger.
-

Does COVID-19 Start With Body Aches?
COVID-19 has symptoms similar to the flu or common cold. Fever, headaches, and body aches are typically the first sign of COVID-19. These pains can come on slowly or appear suddenly.
-

How Long Does Headache Last With COVID-19?
Headache is a potential symptom of COVID-19 and can also occur after getting vaccinated. COVID-19 headaches typically last for a few days, although the duration depends on your age, immune system, and overall health condition. In mild cases of COVID-19, headaches will usually resolve within a few days. However, in more severe cases, mild or moderate headaches may come and go for up to 90 days.
-

How Do You Know if You Have Bronchitis or COVID-19 (Coronavirus)?
What is the difference between bronchitis and COVID-19 (Coronavirus)? Learn how to recognize the symptoms of bronchitis and COVID-19 to help you treat either illness. Bronchitis or “chest cold” refers to the inflammation of the airways (bronchial tubes) in the lungs. Air passes through the lungs within a network of tubules called bronchial tubes. Bronchitis is often associated with persistent, nagging coughs with mucus. Learn more about when a cold becomes bronchitis.
-

What Is the Recommended Pain Reliever for COVID-19?
Acetaminophen (Tylenol), ibuprofen (Advil, Motrin) and naproxen (Aleve) can all be used for pain relief from COVID-19 body aches if they are taken in the recommended doses.
-

What Is Considered to Be Fever for COVID-19?
A body temperature of 100.4 degrees F or higher is generally seen in people with COVID-19, although not everyone who is infected with the virus will develop a fever. Symptoms of COVID-19 may appear 2-14 days after exposure to infection. Other COVID-19 symptoms may include cough, runny nose, body aches, headache, sore throat, difficulty breathing, nausea with or without vomiting, diarrhea, loss of taste, loss of smell, and abdominal pain.
-
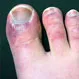
What Are COVID Toes and Fingers?
While less common, COVID-19 can affect your skin. COVID toes and fingers refer to rashes and discoloration on the toes and fingers of people infected with the virus.
-
Is COVID-19 One of the Causes of Pleurisy?
Pleurisy is a painful lung condition that makes it hard to breathe. Learn what causes it, how it's diagnosed, what you can do to treat it, and if COVID-19 causes it.
-

COVID-19 vs. Flu vs. Cold
When you're feeling sick, it can be difficult to distinguish the symptoms of a COVID-19 infection from the symptoms of the common cold or the flu (influenza). While fever is common with the flu and COVID-19, sneezing is typically only associated with colds. Though sore throats are typical with colds, they are uncommon with COVID-19 infections and the flu.
-
How Long Is a COVID-19 Patient Contagious?
People infected with COVID-19 can still be contagious even when they stop feeling sick, so precautionary measures should continue for at least 2 weeks after symptoms disappear and until the COVID-19 test result is negative. Ideally, patients should be quarantined at home or an institution for 2 weeks after the symptoms completely disappear.
-

How Do You Know if You Have a Sinus Infection (Sinusitis) or COVID-19 Coronavirus?
Learn how the signs and symptoms of a sinus infection are different from those caused by COVID-19.
-

Does Your Upper Back Hurt With COVID-19?
COVID-19 can cause upper back pain as well as other body aches. Back pain can even linger months after infection. Learn about what causes back pain with COVID-19.
-

What SpO2 Oxygen Level Is Normal for COVID-19 Patients?
In a patient with COVID-19, SpO2 levels should stay between 92%-96%. Low oxygen levels that drop below this threshold require medical attention.
-

How Long After COVID-19 Symptoms Appear Should I Get Tested?
The CDC recommends that anyone who is exposed to COVID-19 should test four to five days after their suspected exposure.
-

What Is a High Temperature for COVID-19?
COVID-19 infection can cause a fever or high body temperature of 100.4°F or higher.
-

Can Diarrhea Be an Initial Symptom of COVID-19?
COVID-19 has become a common illness that affects many people. Learn the signs of COVID-19, what causes it, how doctors diagnose it, and what you can do to treat it.
-

Which Organ System Is Most Often Affected by COVID-19?
Lungs are the main organs affected by COVID-19; however, the virus can also affect other organs, such as the kidneys, brain, and liver.
-
COVID-19 (Coronavirus) Prevention Tips
COVID-19 is a novel coronavirus that spreads from person to person via infected respiratory droplets. The main symptoms of COVID-19 infection include cough, fever, and shortness of breath. Occasionally, people infected with COVID-19 may experience diarrhea, a sore throat, a runny or stuffy nose, or aches and pains. Avoiding contact with infected people, social distancing, not touching your face, frequent hand washing, cleaning, and disinfecting of frequently touched surfaces can help to reduce your risk of contracting the 2019 novel coronavirus.
-
Can COVID-19 Cause Mediastinal Lymphadenopathy?
COVID-19 can cause mediastinal lymphadenopathy, but it is not considered a typical finding on chest CT scans of patients infected by COVID-19.
-

Does COVID-19 Give You a Stomach Ache?
COVID-19 can cause stomach ache along with other gastrointestinal issues, often the result of liver damage or medications given for treatment.
-
Can a Sore Throat Be the Only Symptom of COVID-19?
Although rare, COVID-19 may present with only sore throat in about 5%-10% of cases. COVID-19-related sore throat is relatively mild and lasts no more than 4-5 days.
-

Can I Get COVID-19 Again?
If you have had COVID-19, can you get it again? Yes, COVID-19 reinfection is rare but possible. Learn what symptoms to look for and how to protect yourself.
-

Does COVID-19 Cause Weird Dreams?
Research shows that the COVID-19 pandemic has had a significant effect on sleep and dream activity in healthy adults.
-
How Soon After the COVID-19 Booster Vaccines Are You Protected?
According to recent studies, it takes about 14 days after receiving the COVID-19 booster vaccine for your immune system to offer protection from the virus.
-

Can I Drink Alcohol Before Getting a COVID-19 Vaccine?
While no scientific evidence exists claiming to avoid alcohol before or after the COVID-19 vaccine, health officials still advise against drinking a week before or after.
-

Can Congestion Be the Only Symptom of COVID-19?
Congestion can be the only symptom of COVID-19 in some cases.
-

How Long Could Fatigue Last After COVID-19 Infection?
Fatigue usually lasts for 2-3 weeks after COVID-19 infection, although some people may experience fatigue for 12 weeks or more after the infection is gone.
-

What Does a COVID-19 Headache Feel Like?
COVID-19 headache may feel like a pulsing, pressing, or stabbing pain.
-

COVID-19 vs. Allergies
Though there is some overlap in allergy and COVID-19 signs and symptoms there are also significant differences. Symptoms that they have in common include headache, fatigue, tiredness, shortness of breath, wheezing, and sore throat. Fever does not occur with allergies but is one of the defining symptoms of COVID-19 infections.
-
Can Babies Get COVID-19?
According to the CDC, it's not common for newborns to be diagnosed with COVID-19. But there have been a few cases of newborns testing positive for the virus.
-
How Does COVID-19 Mainly Spread?
COVID-19 mainly spreads via airborne particles and respiratory droplets formed when an infected person breathes, talks, coughs, or sneezes.
-

What Is the Delta Variant of COVID-19?
Here’s everything you need to know about the Delta variant, why it’s so contagious, and whether COVID-19 vaccines can protect against infection.
Treatment & Diagnosis
- Wuhan Coronavirus FAQs
- Coronavirus COVID-19 Prevention FAQs
- COVID-19 Vaccine Myths and Facts FAQs
- What if I get COVID-19 with Rheumatoid Arthritis?
- What Are Monoclonal Antibody Treatments for COVID-19 Coronavirus?
- Testing Is Key to COVID-19 Recovery for Patients and Economy
- Should I Go to the Dentist During the COVID-19 Pandemic?
- Is the Test for COVID-19 Coronavirus Reliable?
- How Long Can the COVID-19 Coronavirus Survive?
- What if I get COVID-19 with Diabetes?
- What if I Get COVID-19 with Asthma?
Medications & Supplements
Prevention & Wellness
Subscribe to MedicineNet's Daily Health News Newsletter
By clicking Submit, I agree to the MedicineNet's Terms & Conditions & Privacy Policy and understand that I may opt out of MedicineNet's subscriptions at any time.

Report Problems to the Food and Drug Administration
You are encouraged to report negative side effects of prescription drugs to the FDA. Visit the FDA MedWatch website or call 1-800-FDA-1088.
FDA Prescribing Information