|
The specific heat (also called specific heat capacity) is the amount of heat required to change a unit
mass (or unit quantity, such as mole) of a substance by one degree in temperature. Therefore, unlike the
extensive variable heat capacity, which depends on the quantity of material, specific heat is an
intensive variable and has units of energy per mass per degree (or energy per number of moles per degree).
The heat capacity of a substance can differ depending on what extensive variables are held
constant, with the quantity being held constant usually being denoted with a subscript. For example, the specific heat
at constant pressure is commonly denoted 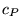 , while the specific heat at constant volume is commonly denoted , while the specific heat at constant volume is commonly denoted  . The specific heat of water at constant atmospheric pressure is . The specific heat of water at constant atmospheric pressure is
 |
(1) |
i.e., 1 calorie is needed per degree Kelvin (or Celsius) of temperature change for 1
gram of liquid water. In fact, the definition of (one of
the several types of) the calorie is the amount of heat needed to change the temperature of 1 g
of water by 1
 at its temperature of maximum density (roughly 3.98° C). at its temperature of maximum density (roughly 3.98° C).
The heat capacity ratio is defined as the ratio of specific heats of a substance at constant pressure and constant
volume,
 |
(2) |
 Heat Capacity, Heat Capacity Ratio, Internal Energy, Latent Heat of Fusion, Latent Heat of Vaporization Heat Capacity, Heat Capacity Ratio, Internal Energy, Latent Heat of Fusion, Latent Heat of Vaporization


O'Hanian, H. C. Physics, Vol. 1. New York: W. W. Norton, pp. 484-487 and 493-496, 1985.
© 1996-2007 Eric W. Weisstein
|