Comparative Study of Synergistic Effects of Antibiotics with Triangular Shaped Silver Nanoparticles, Synthesized Using UV-Light Irradiation, on Staphylococcus aureus and Pseudomonas aeruginosa ()
1. Introduction
Nowadays, among metal nanoparticles, silver is gaining popularity, because it exhibits completely new and improved properties. At nanoscale range, the particle size leads to large surface area per mass where a large number of atoms are in immediate contact and available for reaction. This size, morphology, stability and properties (chemical and physical) of the metal nanoparticles are strongly influenced by the experimental conditions [1] [2] . Hence, the design of a synthesis method in which the size, morphology, stability and properties are controlled, has become a thrust area [3] of research.
It is well known from ancient times that silver nanoparticles are very useful for biomedical applications due to their antibacterial activity. Antibacterial activity of the silver-containing materials can be used, for example, in medicine to reduce infections in burn treatment [4] [5] and arthroplasty [6] as well as to prevent bacteria colonization on prostheses [7] catheters [8] [9] vascular grafts [10] , dental materials [11] , stainless steel materials [12] and human skin [13] [14] . However, with time, the use of silver has reduced as an anti-infection agent due to the advent of antibiotics and other disinfectants and the poorly understood mechanisms of their toxic effects. From studies done by researchers on the synthesis and the antibacterial activity of silver nanoparticles, we find that most of the studies are limited upon interaction of spherical silver nanoparticles with bacterial cell wall [15] [16] . The work done by Pal, et al. [17] was the first study of the effect of shape of nanoparticles on bacterial cell. However, resistance of bacteria to bactericides and antibiotics has increased in recent years. Some antimicrobial agents are extremely irritant and toxic. Hence there is a need to find ways to formulate green and less toxic materials. Plant extracts play an important role in remediation of toxic metals through reduction of the metal ions [18] . Silver nanoparticle are synthesized from various parts of the herbal plants like bark of Citrus limon (Prathna, et al., 2011), cinnamon (Sathishkumar, et al., 2009), tannic acid (Sivaraman, et al., 2009), neem leaves (Tripathi, et al., 2009) and various plant leaves (Song and Kim, 2008).
In the present synthesis route, the author has developed a green route by using tea leaf extract as capping agent, UV-irradiation source as a reducing agent and chitin flakes as stabilizing agent. Black tea leaves contains antioxidants that play a major role in protecting the body against illnesses such as cancer and heart ailments. Compounds of silver like silver oxalate (Ag2C2O4) are photosensitive and yield metallic silver upon the exposure to UV light in the presence of capping agents. Studies [17] [18] show that antimicrobial formulations of silver in the form of nanoparticles could be used as effective bactericidal materials. In the present work, synergistic effect of silver nanoparticles is studied in combination with two standard antibiotics against gram positive and gram negative bacteria. A comparative study between the two standard antibiotics for antibacterial activity and synergistic effects was also conducted.
2. Materials and Method
2.1. Chemicals Used
Materials used for the synthesis of silver nanoparticles are AR grade silver nitrate (AgNO3) and oxalic acid purchased from Merck, India, black tea leaves of red label, Brooke Bond Company, deionized water (Ultra Pure) and chitin flakes of Himedia, CAS No.: 1398-61-4.
2.2. Preparation of Silver Oxalate
Silver oxalate was prepared by mixing 50 ml solution of 0.5 M AgNO3 (Merck, 99%) with 30 ml of 0.5 M oxalic acid (Merck, 99%). The white formed precipitate was filtered washed with distilled water, dried in an air oven for one hour and stored in a dark bottle.
2.3. Preparation of Tea Extract
A 50 ml volumetric flask was filled up to the mark with boiling water and 0.5 gm of tea grains were weighed and transferred to this flask and filtered immediately using Whattman filter paper.
2.4. Synthesis of Colloidal Silver Nanoparticles
Figure 1 shows the experimental set up of the experiment. Experiment was conducted at room temperature. For the synthesis of colloidal solution, 0.02 gm of Ag2C2O4 and 20 ml doubly distilled water was taken in a three neck round bottom flask and stirred for 35 min in dark. Vacuum was created in the flask of the order of 10−3 mm of Hg, followed by UV radiation of strength 256 nm. During the irradiation process no cut-off filter was used. As soon as it is
exposed to UV irradiation, 10 ml of tea extract is added to the solution using a syringe fitted in the cork, placed at one of three necks of the flask. Initially a plane yellow colloidal solution was formed. Then 0.01 gm chitin flakes were added to the solution and process was carried further for a period of 1 hr. Finally, a grey coloured colloidal solution was obtained. The resultant colloid was washed by centrifugation several times.
2.5. Antibacterial Studies
For the comparative study, 1 ml of as-synthesized colloidal solution of silver nanoparticles was taken with 10 mcg of ampicillin and with 10 mcg of gentamicin for 15 minutes separately. Total solution, plated on Soya bean casein digest agar, was incubated at 37˚C for 48 hr. Zone readings were taken for both. The test was conducted at Micro Bio Laboratory Thane (Maharashtra, India).
3. Results and Discussions
Silver oxalate was freshly prepared in the in-house set up. As-synthesised silver oxalate was characterized using XRD (Rigaku Miniflex II). The XRD pattern, Figure 2, of silver oxalate matches with JCPDS file No. 22-1335 indicating primitive monoclinic system. The silver oxalate thus obtained was further characterized using SEM (JSM 6390 A Model). The SEM images of as-synthesised silver oxalate is shown in Figure 3 which indicates irregular shaped silver oxalate particles approximately of size in the range of 500 - 750 nm.
Silver oxalate, thus obtained, was then slowly exposed to UV irradiation along with the addition of black tea leaves extract and chitin flakes. Initially the precursor was exposed for time duration of 30 minutes. The XRD pattern of the precursor, silver oxalate (Figure 4) revealed that when sample was exposed for a time interval of 30 minutes, silver oxalate was partially decomposed to generate silver.
Therefore in order to reduce silver oxalate completely, the exposure time of UV-irradiation was increased to 1 hour. After 1 hr treatment, sample was again characterized using XRD. Figure 5 shows XRD pattern for 1 hour UV irradiation. The graph shows pure peaks of silver which are well in accordance with JCPDS file No. 04- 0783. The diffraction profiles of as-synthesised face centred silver are obviously broadened as compared with bulk silver confirming the formation of silver nanoparticles. Thus for 1 hr duration of UV irradiation, silver oxalate was completely decomposed rendering pure silver nanoparticles.
Silver oxalate decomposes under UV irradiation to give metallic silver and CO2 gas. This is due to the high photosensitivity of Ag2C2O4 [19] . The decomposition of oxalate occurs rapidly under UV radiation to yield metallic Ag as shown in Equation (1) because this decomposition of Ag2C2O4 is thermodynamically favourable due to the suitable reduction potentials of oxalate [20] .
 (1)
(1)
In the presence of UV-irradiation di-anion of silver oxalate gets excited and thus decomposes into CO2, for-

Figure 4. XRD pattern of sample (30 minute of UV-irradiation).

Figure 5. XRD graph of as-synthesised Ag nano particles for 1 hour UV-irradiation.
mation of CO2 can be proved by the appearance of the white precipitate in the baryata solution [21] . To understand the mechanism of the reaction during the decomposition, we see that electron is continuously transferred from silver ion to form silver metal [19] . These silver atoms start to form particles after getting sufficient concentration.
The as-synthesised silver nanoparticles were also characterized using TEM. TEM images shown in Figure 6(a) and Figure 6(b) reveal triangular shaped silver nanoparticles. The surface morphology of as-synthesised Ag nanoparticles may be attributed to the fact that when reduction rate is slow, triangular shaped nanoparticles are synthesized [22] . The average size of nanoparticles obtained was around 50 nm. The size control of nano particles is determined by factors such as precursor concentration, molar ratio between surfactant and precursor, as well as the selective absorption of surfactant to different crystal facets [23] [24] .
Here tea extract acts as capping agent only [25] . UV-vis spectroscopy was done to confirm the formation of nanopaticles and determine the concentration of the colloidal solution. UV-visible graph of the as-synthesized silver nanoparticles is shown in Figure 7.
From the UV-vis graph, it is observed that absorbance peak is obtained at 520nm which is the characteristic peak for triangular shaped silver nanoparticles [26] . Concentration of the colloidal solution for first configuration was calculated using the UV-vis graph and Beer-Lambert law:

where A is the absorbance; ε is molar extinction coefficient; C is the concentration of the solution; l is the dimension of the cuvette. Molar extinction coefficient for silver nanoparticles in water is calculated using Mietheory based power law:

for diameter of Ag np, d > 38 nm [27] .
Here A = 0.98623, l = 1 cm. Thus

Total concentration of the solution is 10 × 11= 110 ppm as for UV-vis spectroscopy the 10 fold dilution of the solution was taken.
4. Antimicrobial Study
Silver nanoparticles have received considerable attention as antimicrobial agents and have been shown to be an
 (a)
(a) (b)
(b)
Figure 6. (a) (b) Tem images of as-synthesised silver nanoparticles for 1 hour reduction time.
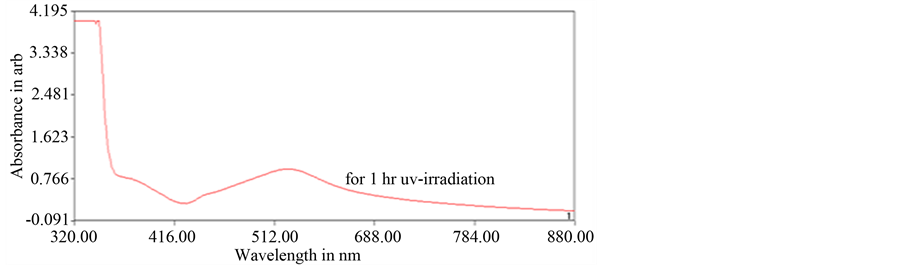
Figure 7. UV-visible spectroscopy of as-synthesised nanoparticles for 1 hour reduction time.
effective antimicrobial agent. The first comparative study on the bactericidal properties of silver particles of different shapes was conducted by Pal et al. [17] . In that work, differently morphological silver particles such as spherical, rod shaped and truncated triangular silver nanoparticles were used to treat microorganism. Interestingly, the truncated triangular nanoparticles displayed the strongest biocidal action, compared with the spherical and rod-shaped nanoparticles. Antibacterial activity of triangular shaped nanoparticle along with antibiotics ampicillin and gentamicin was studied against Staphylococcus aureus and Pseudomonas aeruginosa as triangular nano prism with sharp vertices and edges display higher antibacterial activity in comparison to other shapes of silver nanoparticles which finds these nanoparticles useful for biomedical applications [28] . Also a comparative study of antibacterial effect between the combination of silver nanoparticles with gentamicin and with ampicillin was conducted. Comparative table of zones of inhibition for individual antibiotics and the antibiotics in combination with silver nanoparticles is shown in Table1
Synergistic effects can be clearly observed for from Table1 We can see that in case of P. aeruginosa antibacterial activity is obtained for the combination of silver nanoparticles whereas for the antibiotic alone no activity is observed. Also for the rest of combinations antibacterial activity can be seen. The reason for the reduced size of inhibition zone for combinations as compared to individual antibiotics may be attributed to the improper dispersion of the sample in the petri dish. It is also observed that the inhibition zone size is almost equal against both bacteria for both the antibiotics suggesting existence of same chelation effect between the silver nanoparticles and antibiotics. The mechanism of the bactericidal effect of Ag NPs can be attributed to the fact that they possibly attach to the surface of the cell membrane disturbing permeability and respiration functions of the cell [29] . Sharp vertexes and sharp edges of triangular nano prism would be more toxic in damaging the bacterial cell [28] .

Table 1. Comparative study of effect of silver nanoparticles in combination with two antibiotics for the configuration.
Plate 1 = P1, Plate 2 = P2.
5. Conclusion
In the present work, the author to the best of her knowledge has tried a new way to synthesised silver nano particle of triangular shape using the basic principles of green technology. The average particle size obtained for the nanoparticles was 50 nm. The most important feature of this procedure is that it does not involve usage of hazardous chemicals, which gives an edge over various chemical procedures usually applied for the synthesis of silver nanoparticles. A comparative antibacterial study between as-synthesised silver nanoparticles in combination with two standard antibiotics was analysed for a gram positive and a gram negative bacteria and it was found that the sample in combination with antibiotics is equally effective against both the bacteria. Synergistic effect for the combination in comparison to individual antibiotic ampicillin is clearly visible whereas for rest of the combinations possibly improper dispersion of the sample leads to smaller zone sizes.
Acknowledgements
Author is thankful to the Director, M.A.N.I.T., Bhopal, for the facilities at the institute, AMPRI Bhopal for XRD, for providing XRD facility, HSADL, Bhopal for the TEM facility.
NOTES
*Corresponding author.