Does Minocin (minocycline) cause side effects?
Minocin (minocycline) is a tetracycline antibiotic used to treat infections caused by
- Mycoplasma pneumonia,
- Chlamydia trachomatis,
- Borrelia recurrentis,
- Yersinia pestis,
- Escherichia coli,
- Enterobacter aerogenes,
- Shigella species,
- Acinetobacter species,
- respiratory tract infections caused by Haemophilus influenza and Streptococcus pneumoniae, and
- respiratory tract and urinary tract infections caused by Klebsiella species.
Minocin extended-release tablets are used to treat inflammatory lesions of non-nodular moderate-to-severe acne vulgaris. Minocin is used for treating Rocky Mountain spotted fever, typhus and other infections caused by the typhus group of bacteria, Q fever, rickettsialpox and tick fevers caused by rickettsiae.
Common side effects of Minocin include
- nausea,
- vomiting,
- diarrhea,
- headache,
- fatigue,
- dizziness,
- itching,
- photosensitivity, and
- tooth discoloration.
Serious side effects of Minocin include reduced bone development in children.
Drug interactions of Minocin include anticoagulant medications such as warfarin because it can increase the risk of bleeding and bruising.
- Antacids containing aluminum, calcium, magnesium and iron-containing medications can bind with Minocin, delay the absorption, and reduce the effectiveness of Minocin.
- Minocin should be used with caution with oral contraceptives because it may decrease the effectiveness of oral contraceptives.
Minocin should be avoided in pregnant women because it crosses the placenta and may cause fetal harm.
Minocin is excreted in human milk, and there is potential for serious adverse effects involving the development of teeth and bones in nursing infants. A decision should be made to discontinue Minocin or stop breastfeeding, taking into account the importance of the drug to the mother.
Minocin (minocycline) side effects list for healthcare professionals
Due to oral minocycline’s virtually complete absorption, side effects to the lower bowel, particularly diarrhea, have been infrequent. The following adverse reactions have been observed in patients receiving tetracyclines.
Body as a whole: Fever, and discoloration of secretions.
Gastrointestinal: Anorexia, nausea, vomiting, diarrhea, dyspepsia, stomatitis, glossitis, dysphagia, enamel hypoplasia, enterocolitis, pseudomembranous colitis, pancreatitis, inflammatory lesions (with monilial overgrowth) in the oral and anogenital regions.
Genitourinary: Vulvovaginitis.
Hepatic toxicity: Hyperbilirubinemia, hepatic cholestasis, increases in liver enzymes, fatal hepatic failure, and jaundice. Hepatitis, including autoimmune hepatitis, and liver failure have been reported.
Skin: Alopecia, erythema nodosum, hyperpigmentation of nails, pruritus, toxic epidermal necrolysis, vasculitis, maculopapular rash and erythematous rash. Exfoliative dermatitis has been reported. Fixed drug eruptions, including balanitis, have been reported. Erythema multiforme and Stevens-Johnson syndrome have been reported. Photosensitivity is discussed above. Pigmentation of the skin and mucous membranes has been reported.
Respiratory: Cough, dyspnea, bronchospasm, exacerbation of asthma, and pneumonitis.
Renal toxicity: Interstitial nephritis. Elevations in BUN have been reported and are apparently dose related. Reversible acute renal failure has been reported.
Musculoskeletal: Arthralgia, arthritis, bone discoloration, myalgia, joint stiffness, and joint swelling.
Hypersensitivity reactions: Urticaria, angioneurotic edema, polyarthralgia, anaphylaxis/anaphylactoid reaction (including shock and fatalities), anaphylactoid purpura, myocarditis, pericarditis, exacerbation of systemic lupus erythematosus and pulmonary infiltrates with eosinophilia have been reported. A transient lupus-like syndrome and serum sickness-like reactions also have been reported.
Blood: Agranulocytosis, hemolytic anemia, thrombocytopenia, leukopenia, neutropenia, pancytopenia, and eosinophilia have been reported.
Central Nervous System: Convulsions, dizziness, hypesthesia, paresthesia, sedation, and vertigo. Pseudotumor cerebri (benign intracranial hypertension) in adults and bulging fontanels in infants. Headache has also been reported.
Other: Thyroid cancer has been reported in the post-marketing setting in association with minocycline products. When minocycline therapy is given over prolonged periods, monitoring for signs of thyroid cancer should be considered.
When given over prolonged periods, tetracyclines have been reported to produce brown-black microscopic discoloration of the thyroid gland. Cases of abnormal thyroid function have been reported.
Tooth discoloration in children less than 8 years of age and also, in adults has been reported.
Oral cavity discoloration (including tongue, lip, and gum) has been reported.
Tinnitus and decreased hearing have been reported in patients on MINOCIN (minocycline hydrochloride).
The following syndromes have been reported. In some cases involving these syndromes, death has been reported. As with other serious adverse reactions, if any of these syndromes are recognized, the drug should be discontinued immediately:
Hypersensitivity syndrome consisting of cutaneous reaction (such as rash or exfoliative dermatitis), eosinophilia, and one or more of the following:
- hepatitis,
- pneumonitis,
- nephritis,
- myocarditis, and
- pericarditis.
Fever and lymphadenopathy may be present.
Lupus-like syndrome consisting of positive antinuclear antibody; arthralgia, arthritis, joint stiffness, or joint swelling; and one or more of the following:
- fever,
- myalgia,
- hepatitis,
- rash, and
- vasculitis.
Serum sickness-like syndrome consisting of fever; urticaria or rash; and arthralgia, arthritis, joint stiffness, or joint swelling and lymphadenopathy. Eosinophilia may be present.
To report SUSPECTED ADVERSE REACTIONS, contact Valeant Pharmaceuticals North America LLC at 1-800-321-4576 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
What drugs interact with Minocin (minocycline)?
Because tetracyclines have been shown to depress plasma prothrombin activity, patients who are on anticoagulant therapy may require downward adjustment of their anticoagulant dosage.
Since bacteriostatic drugs may interfere with the bactericidal action of penicillin, it is advisable to avoid giving tetracycline-class drugs in conjunction with penicillin.
Absorption of tetracyclines is impaired by antacids containing aluminum, calcium, or magnesium, and iron-containing preparations.
The concurrent use of tetracycline and methoxyflurane has been reported to result in fatal renal toxicity.
Concurrent use of tetracyclines with oral contraceptives may render oral contraceptives less effective.
Administration of isotretinoin should be avoided shortly before, during, and shortly after minocycline therapy. Each drug alone has been associated with pseudotumor cerebri.
Increased risk of ergotism when ergot alkaloids or their derivatives are given with tetracyclines.
Summary
Minocin (minocycline) is a tetracycline antibiotic used to treat infections and inflammatory lesions of non-nodular moderate-to-severe acne vulgaris. Common side effects of Minocin include nausea, vomiting, diarrhea, headache, fatigue, dizziness, itching, photosensitivity, and tooth discoloration. Serious side effects of Minocin include reduced bone development in children. Minocin should be avoided in pregnant women because it crosses the placenta and may cause fetal harm. Minocin is excreted in human milk, and there is potential for serious adverse effects involving the development of teeth and bones in nursing infants.
Multimedia: Slideshows, Images & Quizzes
-

Bacterial Infections 101: Types, Symptoms, and Treatments
Get more information on bacterial skin infections, which bacteria cause food poisoning, sexually transmitted bacteria, and more....
-

Urinary Tract Infection (UTI) Symptoms, Diagnosis, Medication
Bladder infections can be painful and often require medical treatment. Get the latest information on urinary tract infections...
-

Acne: Foods That Cause and Fight Acne and Pimples
How can you get rid of acne breakouts with nutrition? Does this food cause acne? Milk, chocolate, and seaweed are all considered...
-

Skin Health: How to Get Clear Skin
Acne, pimples, zits and blemishes often appear on the face, back, chest, neck, and shoulders where skin has the most amount of...
-

Bladder Infections: UTI Causes, Symptoms, Treatments
Urinary Tract Infections (UTI's) can happen to anyone. Learn about symptoms, causes and home remedy treatments for bladder and...
-

Acne: Causes, Solutions and Treatments for Adults
Adult acne causes include hormones, medications, makeup, and other things. Adult acne is treated with medications, products, face...
-

Respiratory Illnesses: 13 Types of Lung Infections
Is your cough caused by a cold, flu, pneumonia or something else? Learn causes of respiratory infection like bronchitis,...
-

How to Get Rid of Acne: Medication, Best Treatment, Cystic Acne
What is the best treatment for acne vulgaris? Can food choices influence acne? How can you get rid of blackheads? Learn why it's...
-
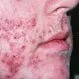
Picture of Cystic Acne
Cystic acne is a type of abscess that is formed when oil ducts become clogged and infected. See a picture of Cystic Acne and...
-

Picture of Erythematous Deep Acne Scars
Acne scarring is a common sequel of severe inflammatory or cystic acne. It can present in a mild or cosmetically disfiguring...
-

Picture of Acne Vulgaris Nodulocystic
The common form of acne, in teens and young adults, that is due to overactivity of the oil (sebaceous) glands in the skin that...
-

Acne (Pimples) Quiz: Test Your Medical IQ
Acne is the most common skin disorder in the world. If you suffer from acne, you are not alone and many treatment options are...
-

Strep (Streptococcal) Throat Infection Quiz: Test Your Infectious Disease IQ
Take the Strep (Streptococcal) Throat Infection Quiz to learn about causes, symptoms, treatments, prevention methods, diagnosis,...
-

Urinary Tract Infection Quiz
How would you know if you had urinary tract infection (UTI)? Take the Urinary Tract Infection in Adult Quiz to learn the causes,...
-
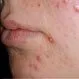
Picture of Acne
Exactly what causes acne? Acne develops when cells and natural oils begin to block up tiny hair follicles in the skin. See a...
-

Picture of Baby Acne
Pink pimples ("neonatal acne") are often caused by exposure in the womb to maternal hormones. See a picture of Baby Acne and...
-

Acne Care Pictures: Skin Care Dos and Don'ts
Explore quick acne cover-ups, dos and don'ts. See solutions on how to best handle pesky pimples and remedies to avoid.
Related Disease Conditions
-

Upper Respiratory Infection (URI)
An upper respiratory infection is a contagious infection of the structures of the upper respiratory tract, which includes the sinuses, nasal passages, pharynx, and larynx. Common causes of an upper respiratory infection include bacteria and viruses such as rhinoviruses, group A streptococci, influenza, respiratory syncytial, whooping cough, diphtheria, and Epstein-Barr. Examples of symptoms of upper respiratory infection include sneezing, sore throat, cough, fever, and nasal congestion. Treatment of upper respiratory infections are based upon the cause. Generally, viral infections are treated symptomatically with over-the-counter (OTC) medication and home remedies.
-

Urinary Tract Infection (UTI)
A urinary tract infection (UTI) is an infection of the bladder, kidneys, ureters, or urethra. E. coli, a type of bacteria that lives in the bowel and near the anus, causes most UTIs. UTI symptoms include pain, abdominal pain, mild fever, urinary urgency, and frequency. Treatment involves a course of antibiotics.
-
Bladder Infection (Cystitis)
Bladder infection is an infection of the bladder, usually caused by bacteria or, rarely, by Candida. Certain people, including females, the elderly, men with enlarged prostates, and those with chronic medical conditions are at increased risk for bladder infection. Bladder infections are treated with antibiotics, but cranberry products and adequate hydration may help prevent bladder infections.
-

Acne
Acne is a localized skin inflammation as a result of the overactivity of oil glands at the base of hair follicles. This inflammation, depending on its location, can take the form of a superficial pustule (contains pus), a pimple, a deeper cyst, congested pores, whiteheads, or blackheads. Treatments vary depending on the severity of the acne.
-
Streptococcal Infections
Group A streptococcal infections are caused by group A Streptococcus, a bacteria that causes a variety of health problems, including strep throat, impetigo, cellulitis, erysipelas, and scarlet fever. There are more than 10 million group A strep infections each year.
-

E. coli (0157:H7) Infection
There are many types of E. coli (Escherichia coli). E. coli can cause urinary tract and bladder infections, or lead to sepsis. E coli O157:H7 (EHEC) causes bloody diarrhea and colitis. Complications of E. coli infection include hemorrhagic diarrhea, hemolytic-uremic syndrome, and thrombotic thrombocytopenic purpura. Symptoms include severe abdominal pain and bloody diarrhea. E coli O157:H7 commonly is due to eating raw or undercooked hamburgers or raw milk or dairy products.
-

Hidradenitis Suppurativa
Hidradenitis suppurativa (HS or acne inversa) is a chronic skin condition that causes painful red abscesses in the groin and armpits that may drain foul-smelling pus. Treatment options include weight loss, smoking cessation, topical antibiotics, and avoidance of tight-fitting underwear. Finasteride and adalimumab may be helpful for those with resistant cases of HS.
-

Yeast Infection vs. Urinary Tract Infection (UTI)
Candida albicans typically causes vaginal yeast infections. Bacterial infections typically cause urinary tract infections (UTIs). Thick white cottage-cheese like vaginal discharge characterizes vaginal yeast infections. Painful, frequent urination characterize urinary tract infections. Antifungal medications treat yeast infections while prescription antibiotics treat UTIs.
-

Urinary Tract Infections in Children
Urinary tract infections (UTIs) are very common in children. Symptoms and signs include fever and abdominal pain. Associated symptoms and signs include flank pain, vomiting, and blood in the urine. Treatment for a UTI involves antibiotic therapy.
Treatment & Diagnosis
- Urinary Tract Infection FAQs
- Acne FAQs
- Strep Streptococcal Throat Infection FAQs
- Accutane (isotretinoin) for Acne linked to birth defects, depression and suicide
- What Is the Difference Between a Bladder Infection vs. UTI?
- Urinary Tract Infection (UTI) Symptoms
- E. coli Infection Facts
- Urinary Tract Infection (UTI) Treatment
Medications & Supplements

Report Problems to the Food and Drug Administration
You are encouraged to report negative side effects of prescription drugs to the FDA. Visit the FDA MedWatch website or call 1-800-FDA-1088.
Professional side effects and drug interactions sections courtesy of the U.S. Food and Drug Administration.