Generic drug: infliximab-axxq
Brand name: Avsola
What is Avsola (infliximab-axxq), and how does it work?
Avsola (infliximab-axxq) is a prescription medicine that is approved for patients with:
- Rheumatoid Arthritis — adults with moderately to severely active rheumatoid arthritis, along with the medicine methotrexate.
- Crohn's Disease — children 6 years and older and adults with Crohn's disease who have not responded well to other medicines.
- Ankylosing Spondylitis.
- Psoriatic Arthritis.
- Plaque Psoriasis — adult patients with plaque psoriasis that is chronic (does not go away), severe, extensive, and/or disabling.
- Ulcerative Colitis — children 6 years and older and adults with moderately to severely active ulcerative colitis who have not responded well to other medicines.
Avsola blocks the action of a protein in your body called tumor necrosis factor-alpha (TNF-alpha). TNF-alpha ismade by your body's immune system. People with certain diseases have too much TNF-alpha that can cause theimmune system to attack normal healthy parts of the body. Avsola can block the damage caused by too much TNF-alpha.
What are the side effects of Avsola?
WARNING
SERIOUS INFECTIONS AND MALIGNANCY
Serious Infections
Patients treated with infliximab products are at increased risk for developing serious infections that may lead to hospitalization or death. Most patients who developed these infections were taking concomitant immunosuppressants such as methotrexate or corticosteroids.
Avsola should be discontinued if a patient develops a serious infection or sepsis.
Reported infections include:
- Active tuberculosis, including reactivation of latent tuberculosis. Patients with tuberculosis have frequently presented with disseminated or extrapulmonary disease. Patients should be tested for latent tuberculosis before Avsola use and during therapy. Treatment for latent infection should be initiated prior to Avsola use.
- Invasive fungal infections, including histoplasmosis, coccidioidomycosis, candidiasis, aspergillosis, blastomycosis, and pneumocystosis. Patients with histoplasmosis or other invasive fungal infections may present with disseminated, rather than localized, disease. Antigen and antibody testing for histoplasmosis may be negative in some patients with active infection. Empiric anti-fungal therapy should be considered in patients at risk for invasive fungal infections who develop severe systemic illness.
- Bacterial, viral and other infections due to opportunistic pathogens, including Legionella and Listeria.
The risks and benefits of treatment with Avsola should be carefully considered prior to initiating therapy in patients with chronic or recurrent infection.
Patients should be closely monitored for the development of signs and symptoms of infection during and after treatment with Avsola, including the possible development of tuberculosis in patients who tested negative for latent tuberculosis infection prior to initiating therapy.
Malignancy
Lymphoma and other malignancies, some fatal, have been reported in children and adolescent patients treated with TNF-blockers, including infliximab products.
Postmarketing cases of hepatosplenic T-cell lymphoma (HSTCL), a rare type of T-cell lymphoma, have been reported in patients treated with TNF-blockers including infliximab products. These cases have had a very aggressive disease course and have been fatal. Almost all patients had received treatment with azathioprine or 6-mercaptopurine concomitantly with a TNF-blocker at or prior to diagnosis. The majority of reported cases have occurred in patients with Crohn’s disease or ulcerative colitis and most were in adolescent and young adult males.
Avsola can cause serious side effects, including:
Serious Infections
- Some patients, especially those 65 years and older have had serious infections while receiving infliximab products, such as Avsola. These serious infections include TB and infections caused by viruses, fungi, or bacteria that have spread throughout the body. Some patients die from these infections. If you get an infection while receiving treatment with Avsola your doctor will treat your infection and may need to stop your Avsola treatment.
- Tell your doctor right away if you have any of the following signs of an infection while receiving or after receiving Avsola:
- Your doctor will examine you for TB and perform a test to see if you have TB. If your doctor feels that you are atrisk for TB, you may be treated with medicine for TB before you begin treatment with Avsola and during treatment with Avsola.
- Even if your TB test is negative, your doctor should carefully monitor you for TB infections while you are receiving Avsola. Patients who had a negative TB skin test before receiving infliximab products have developed active TB.
- If you are a chronic carrier of the hepatitis B virus, the virus can become active while you are being treated with Avsola. In some cases, patients have died as a result of hepatitis B virus being reactivated. Your doctor should do a blood test for hepatitis B virus before you start treatment with Avsola and occasionally while you are being treated. Tell your doctor if you have any of the following symptoms:
Heart Failure
If you have a heart problem called congestive heart failure, your doctor should check you closely while you are receiving Avsola. Your congestive heart failure may get worse while you are receiving Avsola. Be sure to tell your doctor of any new or worse symptoms including:
Treatment with Avsola may need to be stopped if you get new or worse congestive heart failure.
Other Heart Problems
Some patients have experienced a heart attack (some of which led to death), low blood flow to the heart, or abnormal heart rhythm within 24 hours of beginning their infusion of infliximab products. Symptoms may include
- chest discomfort or pain,
- arm pain,
- stomach pain,
- shortness of breath,
- anxiety,
- lightheadedness,
- dizziness,
- fainting,
- sweating,
- nausea,
- vomiting,
- fluttering or pounding in your chest, and/or
- a fast or a slow heartbeat.
Tell your doctor right away if you have any of these symptoms.
Liver Injury
Some patients receiving infliximab products have developed serious liver problems. Tell your doctor if you have:
- jaundice (skin and eyes turning yellow)
- dark brown-colored urine
- pain on the right side of your stomach area (right-sided abdominal pain)
- fever
- extreme tiredness (severe fatigue)
Blood Problems
In some patients receiving infliximab products, the body may not make enough of the blood cells that help fight infections or help stop bleeding. Tell your doctor if you:
- have a fever that does not go away
- look very pale
- bruise or bleed very easily
Nervous System Disorders
Some patients receiving infliximab products have developed problems with their nervous system. Tell your doctor if you have:
- changes in your vision
- numbness or tingling in any part of your body
- weakness in your arms or legs
- seizures
Some patients have experienced a stroke within approximately 24 hours of their infusion with infliximab products. Tell your doctor right away if you have symptoms of a stroke which may include: numbness or weakness of the face, arm or leg, especially on one side of the body; sudden confusion, trouble speaking or understanding; sudden trouble seeing in one or both eyes, sudden trouble walking, dizziness, loss of balance or coordination or a sudden, severe headache.
Allergic Reactions
Some patients have had allergic reactions to infliximab products. Some of these reactions were severe. These reactions can happen while you are getting your Avsola treatment or shortly afterward. Your doctor may need to stop or pause your treatment with Avsola and may give you medicines to treat the allergic reaction. Signs of an allergic reaction can include:
- hives (red, raised, itchy patches of skin)
- high or low blood pressure
- difficulty breathing
- fever
- chest pain
- chills
Some patients treated with infliximab products have had delayed allergic reactions. The delayed reactions occurred 3 to 12 days after receiving treatment with infliximab products. Tell your doctor right away if you have any of these signs of delayed allergic reaction to Avsola:
- fever
- muscle or joint pain
- rash
- swelling of the face and hands
- headache
- difficulty swallowing
- sore throat
Lupus-like Syndrome
Some patients have developed symptoms that are like the symptoms of Lupus. If you develop any of the following symptoms, your doctor may decide to stop your treatment with Avsola.
- chest discomfort or pain that does not go away
- joint pain
- shortness of breath
- rash on the cheeks or arms that gets worse in the sunli>
Psoriasis
Some people receiving infliximab products had new psoriasis or worsening of psoriasis they already had. Tell your doctor if you develop red scaly patches or raised bumps on the skin that are filled with pus. Your doctor may decide to stop your treatment with Avsola.
The most common side effects of infliximab products include:
- respiratory infections, such as sinus
- coughing
- infections and sore throat
- stomach pain
- headache
Infusion reactions can happen up to 2 hours after your infusion of Avsola.
Symptoms of infusion reactions may include:
- fever
- shortness of breath
- chills
- rash
- chest pain
- itching
- low blood pressure or high blood pressure
Children with Crohn's disease showed some differences in side effects compared with adults with Crohn's disease. The side effects that happened more in children were:
- anemia (low red blood cells),
- leukopenia (low white blood cells),
- flushing (redness or blushing),
- viral infections,
- neutropenia (low neutrophils, the white blood cells that fight infection),
- bone fracture,
- bacterial infection and
- allergic reactions of the breathing tract.
Among patients who received infliximab for ulcerative colitis in clinical studies, more children had infections as compared with adults.
Tell your doctor about any side effect that bothers you or does not go away. These are not all of the side effects with Avsola.
Ask your doctor or pharmacist for more information. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.

SLIDESHOW
Inflammatory Bowel Disease (IBD) Causes, Symptoms, Treatment See SlideshowWhat is the dosage for Avsola?
Crohn's Disease
- The recommended dose of Avsola is 5 mg/kg given as an intravenous induction regimen at 0, 2 and 6 weeks followed by a maintenance regimen of 5 mg/kg every 8 weeks thereafter for the treatment of adults with moderately to severely active Crohn's disease or fistulizing Crohn's disease.
- For adult patients who respond and then lose their response, consideration may be given to treatment with 10 mg/kg.
- Patients who do not respond by Week 14 are unlikely to respond with continued dosing and consideration should be given to discontinue Avsola in these patients.
Pediatric Crohn's Disease
- The recommended dose of Avsola for pediatric patients 6 years and older with moderately to severely active Crohn's disease is 5 mg/kg given as an intravenous induction regimen at 0, 2 and 6 weeks followed by a maintenance regimen of 5 mg/kg every 8 weeks.
Ulcerative Colitis
- The recommended dose of Avsola is 5 mg/kg given as an intravenous induction regimen at 0, 2 and 6 weeks followed by a maintenance regimen of 5 mg/kg every 8 weeks thereafter for the treatment of adult patients with moderately to severely active ulcerative colitis.
Pediatric Ulcerative Colitis
- The recommended dose of Avsola for pediatric patients 6 years and older with moderately to severely active ulcerative colitis is 5 mg/kg given as an intravenous induction regimen at 0, 2 and 6 weeks followed by a maintenance regimen of 5 mg/kg every 8 weeks.
Rheumatoid Arthritis
- The recommended dose of Avsola is 3 mg/kg given as an intravenous induction regimen at 0, 2 and 6 weeks followed by a maintenance regimen of 3 mg/kg every 8 weeks thereafter for the treatment of moderately to severely active rheumatoid arthritis. Avsola should be given in combination with methotrexate.
- For patients who have an incomplete response, consideration may be given to adjusting the dose up to 10 mg/kg or treating as often as every 4 weeks bearing in mind that risk of serious infections is increased at higher doses.
Ankylosing Spondylitis
- The recommended dose of Avsola is 5 mg/kg given as an intravenous induction regimen at 0, 2 and 6 weeks followed by a maintenance regimen of 5 mg/kg every 6 weeks thereafter for the treatment of active ankylosing spondylitis.
Psoriatic Arthritis
- The recommended dose of Avsola is 5 mg/kg given as an intravenous induction regimen at 0, 2 and 6 weeks followed by a maintenance regimen of 5 mg/kg every 8 weeks thereafter for the treatment of psoriatic arthritis. Avsola can be used with or without methotrexate.
Plaque Psoriasis
- The recommended dose of Avsola is 5 mg/kg given as an intravenous induction regimen at 0, 2 and 6 weeks followed by a maintenance regimen of 5 mg/kg every 8 weeks thereafter for the treatment of chronic severe (i.e., extensive and/or disabling) plaque psoriasis.
Monitoring To Assess Safety
- Prior to initiating Avsola and periodically during therapy, patients should be evaluated for active tuberculosis and tested for latent infection.
Health News
- More of America's Pets Are Overdosing on Stray Coke, Meth
- GLP-1 Zepbound Is Approved As First Drug For Sleep Apnea
- Feeling Appreciated by Partner is Critical for Caregiver's Mental Health
- Tips for Spending Holiday Time With Family Members Who Live with Dementia
- The Most Therapeutic Kind of Me-Time
 More Health News »
More Health News »
What drugs interact with Avsola?
Use With Anakinra Or Abatacept
- An increased risk of serious infections was seen in clinical studies of other TNFa-blocking agents used in combination with anakinra or abatacept, with no added clinical benefit.
- Because of the nature of the adverse reactions seen with these combinations with TNF-blocker therapy, similar toxicities may also result from the combination of anakinra or abatacept with other TNFa-blocking agents. Therefore, the combination of Avsola and anakinra or abatacept is not recommended.
Use With Tocilizumab
- The use of tocilizumab in combination with biological DMARDs such as TNF antagonists, including Avsola, should be avoided because of the possibility of increased immunosuppression and increased risk of infection.
Use With Other Biological Therapeutics
- The combination of Avsola with other biological therapeutics used to treat the same conditions as Avsola is not recommended.
Methotrexate (MTX) And Other Concomitant Medications
- Specific drug interaction studies, including interactions with MTX, have not been conducted. The majority of patients in rheumatoid arthritis or Crohn's disease clinical studies received one or more concomitant medications. <li>In rheumatoid arthritis, concomitant medications besides MTX were non-steroidal anti-inflammatory agents (NSAIDs), folic acid, corticosteroids and/or narcotics.
- Concomitant Crohn's disease medications were antibiotics, antivirals, corticosteroids, 6-MP/AZA and aminosalicylates.
- In psoriatic arthritis clinical trials, concomitant medications included MTX in approximately half of the patients as well as NSAIDs, folic acid and corticosteroids. Concomitant MTX use may decrease the incidence of anti-drug antibody production and increase infliximab product concentrations.
Immunosuppressants
- Patients with Crohn's disease who received immunosuppressants tended to experience fewer infusion reactions compared to patients on no immunosuppressants.
- Serum infliximab concentrations appeared to be unaffected by baseline use of medications for the treatment of Crohn's disease including corticosteroids, antibiotics (metronidazole or ciprofloxacin) and aminosalicylates.
Cytochrome P450 Substrates
- The formation of CYP450 enzymes may be suppressed by increased levels of cytokines (e.g., TNFα, IL-1, IL-6, IL-10, IFN) during chronic inflammation.
- Therefore, it is expected that for a molecule that antagonizes cytokine activity, such as infliximab products, the formation of CYP450 enzymes could be normalized.
- Upon initiation or discontinuation of Avsola in patients being treated with CYP450 substrates with a narrow therapeutic index, monitoring of the effect (e.g., warfarin) or drug concentration (e.g., cyclosporine or theophylline) is recommended and the individual dose of the drug product may be adjusted as needed.
Live Vaccines/Therapeutic Infectious Agents
- It is recommended that live vaccines not be given concurrently with Avsola. It is also recommended that live vaccines not be given to infants after in utero exposure to infliximab products for at least 6 months following birth.
- It is recommended that therapeutic infectious agents not be given concurrently with Avsola.
Is Avsola safe to use while pregnant or breastfeeding?
- Available data from published literature on the use of infliximab products during pregnancy have not reported a clear association with infliximab products and adverse pregnancy outcomes.
- Infliximab products cross the placenta and infants exposed in utero should not be administered live vaccines for at least 6 months after birth.
- In a development study conducted in mice using an analogous antibody, no evidence of maternal toxicity, embryotoxicity or teratogenicity was observed.
- Available information is insufficient to inform the amount of infliximab products present in human milk, and the effects on the breastfed infant.
- There are no data on the effects of infliximab products on milk production.
- The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for an infliximab product and any potential adverse effects on the breastfed infant from infliximab products or from the underlying maternal condition.
Subscribe to MedicineNet's Daily Health News Newsletter
By clicking Submit, I agree to the MedicineNet's Terms & Conditions & Privacy Policy and understand that I may opt out of MedicineNet's subscriptions at any time.
Summary
Avsola (infliximab-axxq) is a prescription medicine that is approved for patients with rheumatoid arthritis, Crohn's disease, ankylosing spondylitis, psoriatic arthritis, plaque psoriasis, and ulcerative colitis. Serious side effects of Avsola include serious infections, lymphoma and other malignancies, heart failure and other heart problems, liver injury, blood problems, nervous system disorders, allergic reactions, and others.
Multimedia: Slideshows, Images & Quizzes
-

Ulcerative Colitis: Symptoms, Diet, Treatment, Causes
Ulcerative Colitis is a form of inflammatory bowel disease and is slightly different than Crohn's disease. Learn the causes,...
-
Rheumatoid Arthritis: Alternative RA Therapies
Learn which alternative treatments show promise for rheumatoid arthritis.
-

Crohn's Disease: Symptoms, Causes, Diet
What is Crohn's disease? Get more information on this digestive disorder and how Crohn's can affect your diet. Learn more about...
-

Rheumatoid Arthritis: RA Food Myths and Facts
Is there really an RA diet? Learn the truth from WebMD about which foods can ease your symptoms and which you should avoid.
-

Psoriatic Arthritis Symptoms, Treatment, Diagnosis
Psoriatic arthritis pain can be treated. Get more information on the causes, symptoms, diagnosis, and medications for psoriatic...
-

RA Home Remedies
People try all sorts of things to relieve rheumatoid arthritis pain. WebMD debunks some of the common ones and lets you know what...
-

Ulcerative Colitis Quiz: Diet, Symptoms & Treatment
What is ulcerative colitis and what risks are associated with suffering over the long term? Take this Ulcerative Colitis Quiz to...
-

Crohn's Disease Quiz
What causes Crohn's disease? What are the symptoms of Crohn's disease? How is Crohn's treated? Take this quiz to get the facts...
-

Psoriatic Arthritis Quiz: Test Your Medical IQ
How is psoriatic arthritis related to psoriasis? Take this quiz to find out!
-

Rheumatoid Arthritis Exercises: Joint-Friendly Workouts
Regular exercise boosts fitness and helps reverse joint stiffness for people with rheumatoid arthritis (RA). WebMD demonstrates...
-
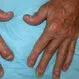
Picture of Psoriatic Arthritis
Psoriatic arthritis is a specific condition in which a person has both psoriasis and arthritis. See a picture of Psoriatic...
Related Disease Conditions
-

Rheumatoid Arthritis (RA)
Rheumatoid arthritis (RA) is an autoimmune disease that causes chronic inflammation of the joints, the tissue around the joints, as well as other organs in the body.
-

Ulcerative Colitis Diet Plan
An ulcerative colitis diet plan can help a person with the disease avoid foods and drinks that trigger flares. There also are foods that can soothe ulcerative colitis symptoms during a flare. Types of ulcerative colitis plans include a high-calorie diet, a lactose-free diet, a low-fat diet, a low-fiber diet (low-residue diet), or a low-salt diet. Self-management of ulcerative colitis using healthy lifestyle habits and a nutrient-rich diet can be effective in the management of the disease. Learn what foods to avoid that aggravate, and what foods help symptoms of the disease and increase bowel inflammation.
-

Ulcerative Colitis
Ulcerative colitis is a chronic inflammation of the colon. Symptoms and signs include abdominal pain, diarrhea, and rectal bleeding. Ulcerative colitis is closely related to Crohn's disease, and together they are referred to as inflammatory bowel disease. Treatment depends upon the type of ulcerative colitis diagnosed.
-

How Long Does an Ulcerative Colitis Flare-Up Last?
An ulcerative colitis flare-up can last a few days or a few weeks and then be followed by a remission that lasts for months or even years. How long a flare-up lasts depends on the severity of the disease, triggers, and medication compliance.
-

Crohn's Disease
Crohn's disease is a chronic inflammatory disease, primarily involving the small and large intestines, but it can affect other parts of the digestive system as well. Abdominal pain, diarrhea, vomiting, fever, and weight loss are common symptoms and signs.
-
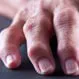
Psoriatic Arthritis
Psoriatic arthritis is a disease that causes skin and joint inflammation. Symptoms of psoriatic arthritis include painful, stiff, and swollen joints, tendinitis, and organ inflammation. Treatment involves anti-inflammatory medications and exercise.
-

What Is the Life Expectancy of Someone with Crohn's Disease?
Crohn’s disease is a chronic condition that causes inflammation in the gut (digestive tract).Crohn’s disease belongs to a group of conditions known as inflammatory bowel disease (IBD). With appropriate management, patients with Crohn’s disease may expect a normal life expectancy and a good quality of life.
-
What Is the Life Expectancy of Someone With Ulcerative Colitis?
Ulcerative colitis (UC) is an inflammatory bowel disease (IBD) that affects the inner lining of the large intestine (large bowel) leading to erosion and ulcers. It is a lifelong illness with no specific cause or cure.
-

Rheumatoid Arthritis vs. Arthritis
Arthritis is a general term used to describe joint disease. Rheumatoid arthritis (RA) is a type of arthritis in which the body’s immune system mistakenly attacks the joints, causing chronic inflammation.
-

Can Rheumatoid Arthritis Be Caused by Stress?
Rheumatoid arthritis can be caused by and result in stress, as well as other conditions such as gastrointestinal problems (IBD).
-

Rheumatoid Arthritis vs. Fibromyalgia
Though rheumatoid arthritis (RA) and fibromyalgia have similar symptoms, RA is an autoimmune disease and fibromyalgia is a chronic pain syndrome. RA symptoms include joint redness, swelling, and pain that lasts more than 6 weeks. Fibromyalgia symptoms include widespread pain, tingling feet or hands, depression, and bowel irritability. Home remedies for both include stress reduction, exercise, and getting enough sleep.
-
Crohn's Disease vs. Ulcerative Colitis
Crohn's disease and ulcerative colitis are diseases that cause inflammation of part of or the entire digestive tract (GI). Crohn's affects the entire GI tract (from the mouth to the anus), while ulcerative colitis or ulcerative colitis only affects the large and small intestines and ilium. Researchers do not know the exact cause of either disease. About 20% of people with Crohn's disease also have a family member with the disease. Researchers believe that certain factors may play a role in causing UC. Both Crohn's disease and ulcerative colitis are a type of inflammatory bowel disease or IBD. Crohn's disease and ulcerative colitis both have similar symptoms and signs, for example, nausea, loss of appetite, fatigue, weight loss, episodic and/or persistent diarrhea, fever, abdominal pain and cramping, rectal bleeding, bloody stools, joint pain and soreness, eye redness, or pain.
-

Is Ulcerative Colitis an Autoimmune Disease?
Ulcerative colitis (UC) is considered to be an autoimmune disease. With autoimmune disorders, your immune system goes awry and attacks your own body instead of defending it from infections and illnesses.
-

What Happens if Crohn’s Is Left Untreated?
Crohn's disease worsens without treatment. When left untreated, Crohn's spreads throughout the intestinal tract, causing severe symptoms and a bleaker outlook to treatment. Colon cancer is more likely to develop in people with untreated Crohn’s in their large intestine.
-

What Does Your Stool Look Like With Ulcerative Colitis?
Ulcerative colitis (UC) is a disease that involves the inner lining of the large bowel. It causes abdominal pain and bleeds due to erosions and ulcers all over the large intestine and rectum.
-

Breastfeeding With Rheumatoid Arthritis
You can breastfeed your baby even if you have rheumatoid arthritis (RA). However, you must always consult your doctor before you start the process.
-

Is Crohn's Disease Contagious?
Crohn's disease, a form of inflammatory bowel disease (IBD), is characterized by symptoms and signs that include diarrhea, fever, weight loss, vomiting, and abdominal pain. Though Crohn's disease is not contagious it can spread throughout a person's gastrointestinal tract. An increase in the above symptoms and signs warrants a visit to a doctor's office.
-

What Does a Crohn’s Disease Attack Feel Like?
Crohn’s disease is an inflammatory bowel disease featuring chronic inflammation of the inner of the gastrointestinal (GI) tract. Patients experience periods of symptomatic relapse and remission. What initiates the autoimmune reaction in Crohn’s disease is unclear, but genetic and environmental factors play roles. Crohn’s disease is a lifelong, progressive disease with no cure.
-
Is Ulcerative Colitis Curable?
Ulcerative colitis is an inflammatory bowel disease (IBD) that affects the inner lining of the large intestine (large bowel or colon) leading to erosion and ulcers. It is also associated with various manifestations outside of the colon, such as inflammation of the eyes, joints, skin, and lungs. Ulcerative colitis is a lifelong illness with no specific cause or cure. Patients have repeated cycles of flare-ups and disappearance of the disease.
Treatment & Diagnosis
- Ulcerative Colitis FAQs
- Crohn's Disease FAQs
- Psoriatic Arthritis FAQs
- Is Crohn's Disease Sexually Transmitted?
- Do Crohn's Patients Get a Specific Type of Arthritis?
- Does Crohn's Disease Cause Arthritis?
- Can Diet and Stress Cause Crohn's Disease?
- Does Stress Cause Ulcerative Colitis?
- Does IBS Cause Crohn's Disease or Ulcerative Colitis?
- Can Crohn's Cause Constipation?
Medications & Supplements

Report Problems to the Food and Drug Administration
You are encouraged to report negative side effects of prescription drugs to the FDA. Visit the FDA MedWatch website or call 1-800-FDA-1088.



