What is Remicade?
Remicade (infliximab) is a monoclonal antibody used to treat Crohn's disease, rheumatoid arthritis, psoriasis, ankylosing spondylitis, and psoriatic arthritis.
Remicade works by blocking the effects of tumor necrosis factor alpha (TNF alpha), a substance made by cells of the body which has an important role in promoting inflammation. By blocking the action of TNF-alpha, infliximab reduces the signs and symptoms of inflammation.
Remicade does not cure Crohn's disease, psoriatic arthritis, or rheumatoid arthritis. Remicade can retard the destruction of joints by rheumatoid arthritis.
Common side effects of Remicade include upper respiratory tract infections, urinary tract infections, cough, rash, back pain, nausea, vomiting, abdominal pain, headache, weakness and fever.
Serious side effects, which could all indicate an allergy to Remicade, include low or high blood pressure, chest pain, difficulty breathing, rash, itching, fever and chills.
Drug interactions of Remicade include with vaccines containing live bacteria or viruses because Remicade may reduce the response of the immune system.
Combining Remicade with anakinra, abatacept, or tocilizumab, drugs that also reduce the response of the immune system, may increase the risk of serious infections.
Use of Remicade in pregnant women has not been adequately evaluated.
It is unknown if Remicade is secreted in breast milk, and, therefore, if there are effects on the nursing infant. Consult your doctor before breastfeeding.
What are the side effects of Remicade?
WARNING
Infliximab should be discontinued if serious reactions occur. Serious infections have been reported with other drugs that block TNF- alpha, and infections have been reported during treatment with infliximab. Therefore, infliximab should not be used in patients with serious infections.
Moreover, infliximab should be discontinued if a serious infection develops during treatment. Testing for tuberculosis (PPD tests for TB) should be doneprior to treatment with infliximab because of reports of reactivation of tuberculosis in patients taking infliximab. Such patients should be treated for tuberculosis.
- Decreased white and red blood cell and decreased platelet counts have been reported with infliximab. Vasculitis (inflammation of the arteries) also has been reported.
- Patients with Crohn's disease or rheumatoid arthritis, particularly patients with highly active disease and/or chronic exposure to immunosuppressant therapies, may be at a higher risk (up to several fold) than the general population for the development of malignant lymphoma.
- More malignancies have been observed in open-label, uncontrolled clinical studies at a rate several-fold higher than expected in the general population. In controlled studies of TNF-alpha blocking agents, including infliximab, more cases of lymphoma and other malignancies have been observed among patients receiving the agents than among control group patients.
What are the common side effects of Remicade?
The most common side effects of infliximab include:
- upper respiratory tract infections,
- urinary tract infections,
- cough,
- rash,
- back pain,
- nausea,
- vomiting,
- abdominal pain,
- headache,
- weakness and
- fever.
What are the serious side effects of Remicade?
Other important side effects include:
- low or high blood pressure,
- chest pain,
- difficulty breathing,
- rash,
- itching,
- fever and
- chills
Reactions listed above could indicate an allergy to the infliximab. They are more common among patients who develop antibodies to infliximab and are less likely to occur in patients who are taking drugs that suppress the immune system, such as methotrexate.
What drugs interact with Remicade?
Use With Anakinra Or Abatacept
- An increased risk of serious infections was seen in clinical studies of other TNFa-blocking agents used in combination with anakinra or abatacept, with no added clinical benefit.
- Because of the nature of the adverse reactions seen with these combinations with TNF- blocker therapy, similar toxicities may also result from the combination of anakinra or abatacept with other TNFa-blocking agents.
- Therefore, the combination of Remicade and anakinra or abatacept is not recommended.
Use With Tocilizumab
- The use of tocilizumab in combination with biological DMARDs such as TNF antagonists, including Remicade, should be avoided because of the possibility of increased immunosuppression and increased risk of infection.
Use With Other Biological Therapeutics
- The combination of Remicade with other biological therapeutics used to treat the same conditions as Remicade is not recommended.
Methotrexate (MTX) And Other Concomitant Medications
- Specific drug interaction studies, including interactions with MTX, have not been conducted. The majority of patients in rheumatoid arthritis or Crohn’s disease clinical studies received one or more concomitant medications.
- In rheumatoid arthritis, concomitant medications besides MTX were
- nonsteroidal anti-inflammatory agents (NSAIDs),
- folic acid,
- corticosteroids and/or
- narcotics.
- Concomitant Crohn’s disease medications were antibiotics, antivirals, corticosteroids, 6-MP/AZA and aminosalicylates.
- In psoriatic arthritis clinical trials, concomitant medications included MTX in approximately half of the patients as well as
- NSAIDs,
- folic acid and
- corticosteroids.
- Concomitant MTX use may decrease the incidence of anti-infliximab antibody production and increase infliximab concentrations.
Immunosuppressants
- Patients with Crohn’s disease who received immunosuppressants tended to experience fewer infusion reactions compared to patients on no immunosuppressants. Serum infliximab concentrations appeared to be unaffected by baseline use of medications for the treatment of Crohn’s disease including corticosteroids, antibiotics (metronidazole or ciprofloxacin) and aminosalicylates.
Cytochrome P450 Substrates
- The formation of CYP450 enzymes may be suppressed by increased levels of cytokines (e.g., TNFα, IL-1, IL-6, IL-10, IFN) during chronic inflammation.
- Therefore, it is expected that for a molecule that antagonizes cytokine activity, such as infliximab, the formation of CYP450 enzymes could be normalized.
- Upon initiation or discontinuation of Remicade in patients being treated with CYP450 substrates with a narrow therapeutic index, monitoring of the effect (e.g., warfarin) or drug concentration (e.g., cyclosporine or theophylline) is recommended and the individual dose of the drug product may be adjusted as needed.
Live Vaccines/Therapeutic Infectious Agents
- It is recommended that live vaccines not be given concurrently with Remicade. It is also recommended that live vaccines not be given to infants after in utero exposure to infliximab for at least 6 months following birth.
- It is recommended that therapeutic infectious agents not be given concurrently with Remicade.
Remicade side effects list for healthcare professionals
Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Adverse Reactions In Adults
The data described herein reflect exposure to Remicade in 4779 adult patients (1304 patients with rheumatoid arthritis, 1106 patients with Crohn’s disease, 202 with ankylosing spondylitis, 293 with psoriatic arthritis, 484 with ulcerative colitis, 1373 with plaque psoriasis, and 17 patients with other conditions), including 2625 patients exposed beyond 30 weeks and 374 exposed beyond 1 year. One of the most-common reasons for discontinuation of treatment was infusion-related reactions (e.g., dyspnea, flushing, headache and rash).
Infusion-related Reactions
- An infusion reaction was defined in clinical trials as any adverse event occurring during an infusion or within 1 hour after an infusion.
- In Phase 3 clinical studies, 18% of Remicade-treated patients experienced an infusion reaction compared to 5% of placebo-treated patients.
- Of infliximab-treated patients who had an infusion reaction during the induction period, 27% experienced an infusion reaction during the maintenance period.
- Of patients who did not have an infusion reaction during the induction period, 9% experienced an infusion reaction during the maintenance period.
- Among all Remicade infusions, 3% were accompanied by nonspecific symptoms such as fever or chills, 1% were accompanied by cardiopulmonary reactions (primarily chest pain, hypotension, hypertension or dyspnea), and <1% were accompanied by pruritus, urticaria, or the combined symptoms of pruritus/urticaria and cardiopulmonary reactions.
- Serious infusion reactions occurred in <1% of patients and included anaphylaxis, convulsions, erythematous rash and hypotension.
- Approximately 3% of patients discontinued Remicade because of infusion reactions, and all patients recovered with treatment and/or discontinuation of the infusion.
- Remicade infusions beyond the initial infusion were not associated with a higher incidence of reactions.
- The infusion reaction rates remained stable in psoriasis through 1 year in psoriasis Study I.
- In psoriasis Study II, the rates were variable over time and somewhat higher following the final infusion than after the initial infusion.
- Across the 3 psoriasis studies, the percent of total infusions resulting in infusion reactions (i.e., an adverse event occurring within 1 hour) was 7% in the 3 mg/kg group, 4% in the 5 mg/kg group, and 1% in the placebo group.
- Patients who became positive for antibodies to infliximab were more likely (approximately two-to three-fold) to have an infusion reaction than were those who were negative.
- Use of concomitant immunosuppressant agents appeared to reduce the frequency of both antibodies to infliximab and infusion reactions.
Infusion Reactions Following Re-administration
- In a clinical trial of patients with moderate to severe psoriasis designed to assess the efficacy of long-term maintenance therapy versus re-treatment with an induction regimen of Remicade following disease flare, 4% (8/219) of patients in the re-treatment therapy arm experienced serious infusion reactions versus <1% (1/222) in the maintenance therapy arm.
- Patients enrolled in this trial did not receive any concomitant immunosuppressant therapy.
- In this study, the majority of serious infusion reactions occurred during the second infusion at Week 2.
- Symptoms included, but were not limited to, dyspnea, urticaria, facial edema, and hypotension.
- In all cases, Remicade treatment was discontinued and/or other treatment instituted with complete resolution of signs and symptoms.
Delayed Reactions/Reactions Following Re-administration
- In psoriasis studies, approximately 1% of Remicade-treated patients experienced a possible delayed hypersensitivity reaction, generally reported as serum sickness or a combination of arthralgia and/or myalgia with fever and/or rash.
- These reactions generally occurred within 2 weeks after repeat infusion.
Infections
- In Remicade clinical studies, treated infections were reported in 36% of Remicade-treated patients (average of 51 weeks of follow-up) and in 25% of placebo-treated patients (average of 37 weeks of follow-up).
- The infections most frequently reported were respiratory tract infections (including sinusitis, pharyngitis, and bronchitis) and urinary tract infections. Among Remicade-treated patients, serious infections included pneumonia, cellulitis, abscess, skin ulceration, sepsis, and bacterial infection.
- In clinical trials, 7 opportunistic infections were reported; 2 cases each of coccidioidomycosis (1 case was fatal) and histoplasmosis (1 case was fatal), and 1 case each of pneumocystosis, nocardiosis and cytomegalovirus.
- Tuberculosis was reported in 14 patients, 4 of whom died due to miliary tuberculosis.
- Other cases of tuberculosis, including disseminated tuberculosis, also have been reported post-marketing.
- Most of these cases of tuberculosis occurred within the first 2 months after initiation of therapy with Remicade and may reflect recrudescence of latent disease.
- In the 1-year placebo-controlled studies RA I and RA II, 5.3% of patients receiving Remicade every 8 weeks with MTX developed serious infections as compared to 3.4% of placebo patients receiving MTX. Of 924 patients receiving Remicade, 1.7% developed pneumonia and 0.4% developed TB, when compared to 0.3% and 0.0% in the placebo arm respectively.
- In a shorter (22-week) placebo-controlled study of 1082 RA patients randomized to receive placebo, 3 mg/kg or 10 mg/kg Remicade infusions at 0, 2, and 6 weeks, followed by every 8 weeks with MTX, serious infections were more frequent in the 10 mg/kg Remicade group (5.3%) than the 3 mg/kg or placebo groups (1.7% in both).
- During the 54-week Crohn’s II Study, 15% of patients with fistulizing Crohn’s disease developed a new fistula-related abscess.
- In Remicade clinical studies in patients with ulcerative colitis, infections treated with antimicrobials were reported in 27% of Remicade-treated patients (average of 41 weeks of follow-up) and in 18% of placebo-treated patients (average 32 weeks of follow-up).
- The types of infections, including serious infections, reported in patients with ulcerative colitis were similar to those reported in other clinical studies.
- The onset of serious infections may be preceded by constitutional symptoms such as fever, chills, weight loss, and fatigue.
- The majority of serious infections, however, may also be preceded by signs or symptoms localized to the site of the infection.
Autoantibodies/Lupus-like Syndrome
- Approximately half of Remicade-treated patients in clinical trials who were antinuclear antibody (ANA) negative at baseline developed a positive ANA during the trial compared with approximately one-fifth of placebo-treated patients.
- Anti-dsDNA antibodies were newly detected in approximately one-fifth of Remicade-treated patients compared with 0% of placebo-treated patients.
- Reports of lupus and lupus-like syndromes, however, remain uncommon.
Malignancies
- In controlled trials, more Remicade-treated patients developed malignancies than placebo-treated patients.
- In a randomized controlled clinical trial exploring the use of Remicade in patients with moderate to severe COPD who were either current smokers or ex-smokers, 157 patients were treated with Remicade at doses similar to those used in rheumatoid arthritis and Crohn’s disease.
- Of these Remicade-treated patients, 9 developed a malignancy, including 1 lymphoma, for a rate of 7.67 cases per 100 patient-years of follow-up (median duration of follow-up 0.8 years; 95% CI 3.51 -14.56).
- There was 1 reported malignancy among 77 control patients for a rate of 1.63 cases per 100 patient-years of follow-up (median duration of follow-up 0.8 years; 95% CI 0.04 -9.10).
- The majority of the malignancies developed in the lung or head and neck.
Patients with Heart Failure
- In a randomized study evaluating Remicade in moderate to severe heart failure (NYHA Class III/IV; left ventricular ejection fraction =35%), 150 patients were randomized to receive treatment with 3 infusions of Remicade 10 mg/kg, 5 mg/kg, or placebo, at 0, 2, and 6 weeks.
- Higher incidences of mortality and hospitalization due to worsening heart failure were observed in patients receiving the 10 mg/kg Remicade dose.
- At 1 year, 8 patients in the 10 mg/kg Remicade group had died compared with 4 deaths each in the 5 mg/kg Remicade and the placebo groups.
- There were trends toward increased dyspnea, hypotension, angina, and dizziness in both the 10 mg/kg and 5 mg/kg Remicade treatment groups, versus placebo.
- Remicade has not been studied in patients with mild heart failure (NYHA Class I/II).
Immunogenicity
- Treatment with Remicade can be associated with the development of antibodies to infliximab.
- An enzyme immunoassay (EIA) method was originally used to measure antiinfliximab antibodies in clinical studies of Remicade.
- The EIA method is subject to interference by serum infliximab, possibly resulting in an underestimation of the rate of patient antibody formation.
- A separate, drug-tolerant electrochemiluminescence immunoassay (ECLIA) method for detecting antibodies to infliximab was subsequently developed and validated.
- This method is 60-fold more sensitive than the original EIA.
- With the ECLIA method, all clinical samples can be classified as either positive or negative for antibodies to infliximab without the need for the inconclusive category.
- The incidence of antibodies to infliximab was based on the original EIA method in all clinical studies of Remicade except for the Phase 3 study in pediatric patients with ulcerative colitis where the incidence of antibodies to infliximab was detected using both the EIA and ECLIA methods.
- The incidence of antibodies to infliximab in patients given a 3-dose induction regimen followed by maintenance dosing was approximately 10% as assessed through 1 to 2 years of Remicade treatment.
- A higher incidence of antibodies to infliximab was observed in Crohn’s disease patients receiving Remicade after drug-free intervals >16 weeks.
- In a study of psoriatic arthritis in which 191 patients received 5 mg/kg with or without MTX, antibodies to infliximab occurred in 15% of patients.
- The majority of antibody-positive patients had low titers.
- Patients who were antibody-positive were more likely to have higher rates of clearance, reduced efficacy and to experience an infusion reaction than were patients who were antibody negative.
- Antibody development was lower among rheumatoid arthritis and Crohn’s disease patients receiving immunosuppressant therapies such as 6-MP/AZA or MTX.
- In the psoriasis Study II, which included both the 5 mg/kg and 3 mg/kg doses, antibodies were observed in 36% of patients treated with 5 mg/kg every 8 weeks for 1 year, and in 51% of patients treated with 3 mg/kg every 8 weeks for 1 year.
- In the psoriasis Study III, which also included both the 5 mg/kg and 3 mg/kg doses, antibodies were observed in 20% of patients treated with 5 mg/kg induction (weeks 0, 2 and 6), and in 27% of patients treated with 3 mg/kg induction.
- Despite the increase in antibody formation, the infusion reaction rates in Studies I and II in patients treated with 5 mg/kg induction followed by every 8 week maintenance for 1 year and in Study III in patients treated with 5 mg/kg induction (14.1%23.0%) and serious infusion reaction rates (<1%) were similar to those observed in other study populations.
- The clinical significance of apparent increased immunogenicity on efficacy and infusion reactions in psoriasis patients as compared to patients with other diseases treated with Remicade over the long term is not known.
- The data reflect the percentage of patients whose test results were positive for antibodies to infliximab in an immunoassay, and they are highly dependent on the sensitivity and specificity of the assay.
- Additionally, the observed incidence of antibody positivity in an assay may be influenced by several factors including sample handling, timing of sample collection, concomitant medication, and underlying disease.
- For these reasons, comparison of the incidence of antibodies to infliximab with the incidence of antibodies to other products may be misleading.
Hepatotoxicity
- Severe liver injury, including acute liver failure and autoimmune hepatitis, has been reported in patients receiving Remicade.
- Reactivation of hepatitis B virus has occurred in patients receiving TNF-blocking agents, including Remicade, who are chronic carriers of this virus.
- In clinical trials in rheumatoid arthritis, Crohn’s disease, ulcerative colitis, ankylosing spondylitis, plaque psoriasis, and psoriatic arthritis, elevations of aminotransferases were observed (ALT more common than AST) in a greater proportion of patients receiving Remicade than in controls (Table 1), both when Remicade was given as monotherapy and when it was used in combination with other immunosuppressive agents.
- In general, patients who developed ALT and AST elevations were asymptomatic, and the abnormalities decreased or resolved with either continuation or discontinuation of Remicade, or modification of concomitant medications.
Table 1: Proportion of patients with elevated ALT in clinical trials
| Proportion of patients with elevated ALT | ||||||
| >1 to <3 x ULN | ≥3 x ULN | ≥5 x ULN | ||||
| Placebo | Remicade | Placebo | Remicade | Placebo | Remicade | |
| Rheumatoid arthritisa | 24% | 34% | 3% | 4% | <1% | <1% |
| Crohn’s diseaseb | 34% | 39% | 4% | 5% | 0% | 2% |
| Ulcerative colitisc | 12% | 17% | 1% | 2% | <1% | <1% |
| Ankylosing spondylitisd | 15% | 51% | 0% | 10% | 0% | 4% |
| Psoriatic arthritise | 16% | 50% | 0% | 7% | 0% | 2% |
| Plaque psoriasisf | 24% | 49% | <1% | 8% | 0% | 3% |
| a Placebo patients received methotrexate while Remicade patients received both Remicade and methotrexate. Median follow-up was 58 weeks. b Placebo patients in the 2 Phase 3 trials in Crohn’s disease received an initial dose of 5 mg/kg Remicade at study start and were on placebo in the maintenance phase. Patients who were randomized to the placebo maintenance group and then later crossed over to Remicade are included in the Remicade group in ALT analysis. Median follow-up was 54 weeks. c Median follow-up was 30 weeks. Specifically, the median duration of follow-up was 30 weeks for placebo and 31 weeks for Remicade. d Median follow-up was 24 weeks for the placebo group and 102 weeks for the Remicade group. e Median follow-up was 39 weeks for the Remicade group and 18 weeks for the placebo group. f ALT values are obtained in 2 Phase 3 psoriasis studies with median follow-up of 50 weeks for Remicade and 16 weeks for placebo. |
||||||
Adverse Reactions in Psoriasis Studies
- During the placebo-controlled portion across the 3 clinical trials up to Week 16, the proportion of patients who experienced at least 1 serious adverse reaction (SAE; defined as resulting in death, life threatening, requires hospitalization, or persistent or significant disability/incapacity) was 0.5% in the 3 mg/kg Remicade group, 1.9% in the placebo group, and 1.6% in the 5 mg/kg Remicade group.
- Among patients in the 2 Phase 3 studies, 12.4% of patients receiving Remicade 5 mg/kg every 8 weeks through 1 year of maintenance treatment experienced at least 1 SAE in Study I.
- In Study II, 4.1% and 4.7% of patients receiving Remicade 3 mg/kg and 5 mg/kg every 8 weeks, respectively, through 1 year of maintenance treatment experienced at least 1 SAE.
- One death due to bacterial sepsis occurred 25 days after the second infusion of 5 mg/kg Remicade.
- Serious infections included sepsis, and abscesses.
- In Study I, 2.7% of patients receiving Remicade 5 mg/kg every 8 weeks through 1 year of maintenance treatment experienced at least 1 serious infection.
- In Study II, 1.0% and 1.3% of patients receiving Remicade 3 mg/kg and 5 mg/kg, respectively, through 1 year of treatment experienced at least 1 serious infection.
- The most common serious infection (requiring hospitalization) was abscess (skin, throat, and peri-rectal) reported by 5 (0.7%) patients in the 5 mg/kg Remicade group.
- Two active cases of tuberculosis were reported: 6 weeks and 34 weeks after starting Remicade.
- In the placebo-controlled portion of the psoriasis studies, 7 of 1123 patients who received Remicade at any dose were diagnosed with at least one NMSC compared to 0 of 334 patients who received placebo.
- In the psoriasis studies, 1% (15/1373) of patients experienced serum sickness or a combination of arthralgia and/or myalgia with fever, and/or rash, usually early in the treatment course.
- Of these patients, 6 required hospitalization due to fever, severe myalgia, arthralgia, swollen joints, and immobility.
Other Adverse Reactions
- Safety data are available from 4779 Remicade-treated adult patients, including
- 1304 with rheumatoid arthritis,
- 1106 with Crohn’s disease,
- 484 with ulcerative colitis,
- 202 with ankylosing spondylitis,
- 293 with psoriatic arthritis,
- 1373 with plaque psoriasis and
- 17 with other conditions.
- Adverse reactions reported in ≥5% of all patients with rheumatoid arthritis receiving 4 or more infusions are in Table 2.
- The types and frequencies of adverse reactions observed were similar in Remicade-treated rheumatoid arthritis, ankylosing spondylitis, psoriatic arthritis, plaque psoriasis and Crohn’s disease patients except for abdominal pain, which occurred in 26% of Remicade-treated patients with Crohn’s disease.
- In the Crohn’s disease studies, there were insufficient numbers and duration of follow-up for patients who never received Remicade to provide meaningful comparisons.
Table 2: Adverse reactions occurring in 5% or more of patients receiving 4 or more infusions for rheumatoid arthritis
| Placebo | Remicade | |
| (n=350) | (n=1129) | |
| Average weeks of follow-up | 59 | 66 |
| Gastrointestinal | ||
| Nausea | 20% | 21% |
| Abdominal pain | 8% | 12% |
| Diarrhea | 12% | 12% |
| Dyspepsia | 7% | 10% |
| Respiratory | ||
| Upper respiratory tract infection | 25% | 32% |
| Sinusitis | 8% | 14% |
| Pharyngitis | 8% | 12% |
| Coughing | 8% | 12% |
| Bronchitis | 9% | 10% |
| Skin and appendages disorders | ||
| Rash | 5% | 10% |
| Pruritus | 2% | 7% |
| Body as a whole-general disorders | ||
| Fatigue | 7% | 9% |
| Pain | 7% | 8% |
| Resistance mechanism disorders | ||
| Fever | 4% | 7% |
| Moniliasis | 3% | 5% |
| Central and peripheral nervous system disorders | ||
| Headache | 14% | 18% |
| Musculoskeletal system disorders | ||
| Arthralgia | 7% | 8% |
| Urinary system disorders | ||
| Urinary tract infection | 6% | 8% |
| Cardiovascular disorders, general | ||
| Hypertension | 5% | 7% |
The most common serious adverse reactions observed in clinical trials were infections. Other serious, medically relevant adverse reactions ≥0.2% or clinically significant adverse reactions by body system were as follows:
- Body as a whole: allergic reaction, edema
- Blood: pancytopenia
- Cardiovascular: hypotension
- Gastrointestinal: constipation, intestinal obstruction
- Central and Peripheral Nervous: dizziness
- Heart Rate and Rhythm: bradycardia
- Liver and Biliary: hepatitis
- Metabolic and Nutritional: dehydration
- Platelet, Bleeding and Clotting: thrombocytopenia
- Neoplasms: lymphoma
- Red Blood Cell: anemia, hemolytic anemia
- Resistance Mechanism: cellulitis, sepsis, serum sickness, sarcoidosis
- Respiratory: lower respiratory tract infection (including pneumonia), pleurisy, pulmonary edema
- Skin and Appendages: increased sweating
- Vascular (Extracardiac): thrombophlebitis
- White Cell and Reticuloendothelial: leukopenia, lymphadenopathy
Adverse Reactions In Pediatric Patients
Pediatric Crohn’s Disease
- There were some differences in the adverse reactions observed in the pediatric patients receiving Remicade compared to those observed in adults with Crohn’s disease. These differences are discussed in the following paragraphs.
- The following adverse reactions were reported more commonly in 103 randomized pediatric Crohn’s disease patients administered 5 mg/kg Remicade through 54 weeks than in 385 adult Crohn’s disease patients receiving a similar treatment regimen:
- anemia (11%),
- leukopenia (9%),
- flushing (9%),
- viral infection (8%),
- neutropenia (7%),
- bone fracture (7%),
- bacterial infection (6%), and
- respiratory tract allergic reaction (6%).
- Infections were reported in 56% of randomized pediatric patients in Study Peds Crohn’s and in 50% of adult patients in Study Crohn’s I.
- In Study Peds Crohn’s, infections were reported more frequently for patients who received every 8-week as opposed to every 12-week infusions (74% and 38%, respectively), while serious infections were reported for 3 patients in the every 8-week and 4 patients in the every 12-week maintenance treatment group.
- The most commonly reported infections were upper respiratory tract infection and pharyngitis, and the most commonly reported serious infection was abscess.
- Pneumonia was reported for 3 patients, (2 in the every 8-week and 1 in the every 12-week maintenance treatment groups).
- Herpes zoster was reported for 2 patients in the every 8-week maintenance treatment group.
- In Study Peds Crohn’s, 18% of randomized patients experienced 1 or more infusion reactions, with no notable difference between treatment groups.
- Of the 112 patients in Study Peds Crohn’s, there were no serious infusion reactions, and 2 patients had non-serious anaphylactoid reactions.
- In Study Peds Crohn’s, in which all patients received stable doses of 6-MP, AZA, or MTX, excluding inconclusive samples, 3 of 24 patients had antibodies to infliximab.
- Although 105 patients were tested for antibodies to infliximab, 81 patients were classified as inconclusive because they could not be ruled as negative due to assay interference by the presence of infliximab in the sample.
- Elevations of ALT up to 3 times the upper limit of normal (ULN) were seen in 18% of pediatric patients in Crohn’s disease clinical trials; 4% had ALT elevations .3 x ULN, and 1% had elevations ≥5 x ULN. (Median follow-up was 53 weeks.)
Pediatric Ulcerative Colitis
- Overall, the adverse reactions reported in the pediatric ulcerative colitis trial and adult ulcerative colitis (Study UC I and Study UC II) studies were generally consistent.
- In a pediatric UC trial, the most common adverse reactions were
- upper respiratory tract infection,
- pharyngitis,
- abdominal pain,
- fever, and
- headache.
- Infections were reported in 31 (52%) of 60 treated patients in the pediatric UC trial and 22 (37%) required oral or parenteral antimicrobial treatment.
- The proportion of patients with infections in the pediatric UC trial was similar to that in the pediatric Crohn’s disease study (Study Peds Crohn’s) but higher than the proportion in the adults’ ulcerative colitis studies (Study UC I and Study UC II).
- The overall incidence of infections in the pediatric UC trial was 13/22 (59%) in the every 8 week maintenance treatment group.
- Upper respiratory tract infection (7/60 [12%]) and pharyngitis (5/60 [8%]) were the most frequently reported respiratory system infections.
- Serious infections were reported in 12% (7/60) of all treated patients.
- In the pediatric UC trial, 58 patients were evaluated for antibodies to infliximab using the EIA as well as the drug-tolerant ECLIA.
- With the EIA, 4 of 58 (7%) patients had antibodies to infliximab.
- With the ECLIA, 30 of 58 (52%) patients had antibodies to infliximab.
- The higher incidence of antibodies to infliximab by the ECLIA method was due to the 60-fold higher sensitivity compared to the EIA method.
- While EIA-positive patients generally had undetectable trough infliximab concentrations, ECLIA-positive patients could have detectable trough concentrations of infliximab because the ECLIA assay is more sensitive and drug-tolerant.
- Elevations of ALT up to 3 times the upper limit of normal (ULN) were seen in 17% (10/60) of pediatric patients in the pediatric UC trial; 7% (4/60) had ALT elevations ≥3 x ULN, and 2% (1/60) had elevations ≥5 x ULN (median follow-up was 49 weeks).
- Overall, 8 of 60 (13%) treated patients experienced one or more infusion reactions, including 4 of 22 (18%) patients in the every 8-week treatment maintenance group. No serious infusion reactions were reported.
- In the pediatric UC trial, 45 patients were in the 12 to 17 year age group and 15 in the 6 to 11 year age group.
- The numbers of patients in each subgroup are too small to make any definitive conclusions about the effect of age on safety events.
- There were higher proportions of patients with serious adverse events (40% vs. 18%) and discontinuation due to adverse events (40% vs. 16%) in the younger age group than in the older age group.
- While the proportion of patients with infections was also higher in the younger age group (60% vs. 49%), for serious infections, the proportions were similar in the two age groups (13% in the 6 to 11 year age group vs. 11% in the 12 to 17 year age group).
- Overall proportions of adverse reactions, including infusion reactions, were similar between the 6 to 11 and 12 to 17 year age groups (13%).
Postmarketing Experience
- Adverse reactions have been identified during post approval use of Remicade in adult and pediatric patients.
- Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
- The following adverse reactions, some with fatal outcome, have been reported during post-approval use of Remicade:
- neutropenia,
- agranulocytosis (including infants exposed in utero to infliximab),
- interstitial lung disease (including pulmonary fibrosis/interstitial pneumonitis and rapidly progressive disease),
- idiopathic thrombocytopenic purpura,
- thrombotic thrombocytopenic purpura,
- pericardial effusion,
- systemic and cutaneous vasculitis,
- erythema multiforme,
- Stevens-Johnson Syndrome,
- toxic epidermal necrolysis,
- peripheral demyelinating disorders (such as Guillain-Barre syndrome, chronic inflammatory demyelinating polyneuropathy, and multifocal motor neuropathy),
- new onset and worsening psoriasis (all subtypes including pustular, primarily palmoplantar),
- transverse myelitis, and neuropathies (additional neurologic reactions have also been observed),
- acute liver failure,
- jaundice,
- hepatitis, and cholestasis,
- serious infections, malignancies, including melanoma, Merkel cell carcinoma, and cervical cancer and vaccine breakthrough infection including bovine tuberculosis (disseminated BCG infection) following vaccination in an infant exposed in utero to infliximab.
Infusion-Related Reactions
- In post-marketing experience, cases of anaphylactic reactions, including laryngeal/pharyngeal edema and severe bronchospasm, and seizure have been associated with Remicade administration.
- Cases of transient visual loss have been reported in association with Remicade during or within 2 hours of infusion.
- Cerebrovascular accidents, myocardial ischemia/infarction (some fatal), and arrhythmia occurring within 24 hours of initiation of infusion have also been reported.
Adverse Reactions In Pediatric Patients
- The following serious adverse reactions have been reported in the post-marketing experience in children:
- infections (some fatal) including opportunistic infections and tuberculosis,
- infusion reactions, and
- hypersensitivity reactions.
- Serious adverse reactions in the post-marketing experience with Remicade in the pediatric population have also included malignancies, including
- hepatosplenic T-cell lymphomas,
- transient hepatic enzyme abnormalities,
- lupus-like syndromes, and
- the development of autoantibodies.
Summary
Remicade (infliximab) is a monoclonal antibody used to treat Crohn's disease, rheumatoid arthritis, psoriasis, ankylosing spondylitis, and psoriatic arthritis. Common side effects of Remicade include upper respiratory tract infections, urinary tract infections, cough, rash, back pain, nausea, vomiting, abdominal pain, headache, weakness and fever. Use of Remicade in pregnant women has not been adequately evaluated. It is unknown if Remicade is secreted in breast milk, and, therefore, if there are effects on the nursing infant.
Multimedia: Slideshows, Images & Quizzes
-

What Is Rheumatoid Arthritis (RA)? Symptoms, Treatment, Diagnosis
What is rheumatoid arthritis (RA)? Learn about treatment, diagnosis, and the symptoms of juvenile rheumatoid arthritis. Discover...
-

Crohn's Disease: Symptoms, Causes, Diet
What is Crohn's disease? Get more information on this digestive disorder and how Crohn's can affect your diet. Learn more about...
-

Psoriatic Arthritis Symptoms, Treatment, Diagnosis
Psoriatic arthritis pain can be treated. Get more information on the causes, symptoms, diagnosis, and medications for psoriatic...
-

Crohn's Disease Quiz
What causes Crohn's disease? What are the symptoms of Crohn's disease? How is Crohn's treated? Take this quiz to get the facts...
-

Psoriatic Arthritis Quiz: Test Your Medical IQ
How is psoriatic arthritis related to psoriasis? Take this quiz to find out!
-

Rheumatoid Arthritis Quiz: What is Rheumatoid Arthritis?
How is rheumatoid arthritis different from other forms of arthritis, such as osteoarthritis and gout? Take the Rheumatoid...
-

Rheumatoid Arthritis Exercises: Joint-Friendly Workouts
Regular exercise boosts fitness and helps reverse joint stiffness for people with rheumatoid arthritis (RA). WebMD demonstrates...
-
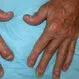
Picture of Psoriatic Arthritis
Psoriatic arthritis is a specific condition in which a person has both psoriasis and arthritis. See a picture of Psoriatic...
-
Arthritis: Causes and Treatment for Joint Stiffness and Pain
Arthritis and injuries can leave your joints swollen, tender, and damaged. Discover treatments for morning stiffness, sore...
-

Arthritis: 16 Bad Habits That Cause Joint Pain
Being overweight, wearing uncomfortable shoes, or carrying a heavy purse can make joint pain and arthritis symptoms worse. Some...
-

Tips for Healthy Joints: Exercise, Nutrition, & More
Dealing with joint pain and arthritis? Learn why weight matters--and why NOT to stretch before exercise. See these solutions for...
-

Famous Faces With Rheumatoid Arthritis
Learn more about the famous faces of rheumatoid arthritis such as Lucille Ball, Glenn Frey, and more.
-

Fun With Kids? Don't Let Arthritis Stop You
You can still have lots of fun with children despite arthritis. Our experts uncover ways to spend time with your kids or...
Related Disease Conditions
-

Rheumatoid Arthritis (RA)
Rheumatoid arthritis (RA) is an autoimmune disease that causes chronic inflammation of the joints, the tissue around the joints, as well as other organs in the body.
-

Arthritis (Joint Inflammation)
Arthritis is inflammation of one or more joints. When joints are inflamed they can develop stiffness, warmth, swelling, redness and pain. There are over 100 types of arthritis, including osteoarthritis, rheumatoid arthritis, ankylosing spondylitis, psoriatic arthritis, and gout.
-

Crohn's Disease
Crohn's disease is a chronic inflammatory disease, primarily involving the small and large intestines, but it can affect other parts of the digestive system as well. Abdominal pain, diarrhea, vomiting, fever, and weight loss are common symptoms and signs.
-
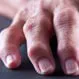
Psoriatic Arthritis
Psoriatic arthritis is a disease that causes skin and joint inflammation. Symptoms of psoriatic arthritis include painful, stiff, and swollen joints, tendinitis, and organ inflammation. Treatment involves anti-inflammatory medications and exercise.
-

What Is the Life Expectancy of Someone with Crohn's Disease?
Crohn’s disease is a chronic condition that causes inflammation in the gut (digestive tract).Crohn’s disease belongs to a group of conditions known as inflammatory bowel disease (IBD). With appropriate management, patients with Crohn’s disease may expect a normal life expectancy and a good quality of life.
-

Rheumatoid Arthritis vs. Arthritis
Arthritis is a general term used to describe joint disease. Rheumatoid arthritis (RA) is a type of arthritis in which the body’s immune system mistakenly attacks the joints, causing chronic inflammation.
-

Can Rheumatoid Arthritis Be Caused by Stress?
Rheumatoid arthritis can be caused by and result in stress, as well as other conditions such as gastrointestinal problems (IBD).
-
Rheumatoid Arthritis vs. Lupus: Differences and Similarities
Rheumatoid arthritis (RA) and lupus are two varieties of autoimmune diseases that cause flare-ups. While RA attacks the immune system on the joints, lupus involves many other parts of the body besides the joints. Common RA symptoms involve warm, swollen, and painful joints; morning stiffness in the joints or stiffness after inactivity, joint deformity, fever, fatigue, etc. Lupus symptoms include Malar rash (butterfly-shaped rash involving the cheeks and bridge of the nose), fever, joint pain in the absence of joint deformity, etc.
-

Rheumatoid Arthritis vs. Fibromyalgia
Though rheumatoid arthritis (RA) and fibromyalgia have similar symptoms, RA is an autoimmune disease and fibromyalgia is a chronic pain syndrome. RA symptoms include joint redness, swelling, and pain that lasts more than 6 weeks. Fibromyalgia symptoms include widespread pain, tingling feet or hands, depression, and bowel irritability. Home remedies for both include stress reduction, exercise, and getting enough sleep.
-
Crohn's Disease vs. Ulcerative Colitis
Crohn's disease and ulcerative colitis are diseases that cause inflammation of part of or the entire digestive tract (GI). Crohn's affects the entire GI tract (from the mouth to the anus), while ulcerative colitis or ulcerative colitis only affects the large and small intestines and ilium. Researchers do not know the exact cause of either disease. About 20% of people with Crohn's disease also have a family member with the disease. Researchers believe that certain factors may play a role in causing UC. Both Crohn's disease and ulcerative colitis are a type of inflammatory bowel disease or IBD. Crohn's disease and ulcerative colitis both have similar symptoms and signs, for example, nausea, loss of appetite, fatigue, weight loss, episodic and/or persistent diarrhea, fever, abdominal pain and cramping, rectal bleeding, bloody stools, joint pain and soreness, eye redness, or pain.
-

Breastfeeding With Rheumatoid Arthritis
You can breastfeed your baby even if you have rheumatoid arthritis (RA). However, you must always consult your doctor before you start the process.
-

Is Crohn's Disease Contagious?
Crohn's disease, a form of inflammatory bowel disease (IBD), is characterized by symptoms and signs that include diarrhea, fever, weight loss, vomiting, and abdominal pain. Though Crohn's disease is not contagious it can spread throughout a person's gastrointestinal tract. An increase in the above symptoms and signs warrants a visit to a doctor's office.
Treatment & Diagnosis
- Rheumatoid Arthritis FAQs
- Crohn's Disease FAQs
- Psoriatic Arthritis FAQs
- Will Rheumatoid Arthritis Nodules Go Away?
- What if I get COVID-19 with Rheumatoid Arthritis?
- Are Corticosteroids Safe for Pregnant and Nursing Women with Rheumatoid Arthritis?
- Psoriatic Arthritis and Ankylosing Spondylitis - 2001 Meeting
- Arava Approved For Rheumatoid Arthritis
- 5 Surprising Facts About Rheumatoid Arthritis
- Is Crohn's Disease Sexually Transmitted?
- What Not to Eat When You Have Arthritis
- Can Milk Allergy Cause Rheumatoid Arthritis?
- Do Crohn's Patients Get a Specific Type of Arthritis?
- Does Crohn's Disease Cause Arthritis?
- Can Diet and Stress Cause Crohn's Disease?
- Does IBS Cause Crohn's Disease or Ulcerative Colitis?
- Can Crohn's Cause Constipation?
Medications & Supplements

Report Problems to the Food and Drug Administration
You are encouraged to report negative side effects of prescription drugs to the FDA. Visit the FDA MedWatch website or call 1-800-FDA-1088.
Professional side effects and drug interactions sections courtesy of the U.S. Food and Drug Administration.